Inhibition of lipid peroxidation and free radical scavenging activities of methanolic leaf extract of Psidium guajava
Keywords:
Psidium guajava, antioxidant, phenol, flavonoids, paraoxonaseAbstract
Objectives: Studies have linked the generation of free radicals with the incidence of degenerative diseases. Antioxidants from plant origin have been proved to play a major role in mitigating against free radicals-induced oxidative damage. This study aimed at assessing the in-vitro and in-vivo antioxidant capability of Psidium guajava leaf
Methods: The leaves were collected and extracted with 70% methanol. Total phenolic, and flavonoids contents, 2,2-diphenyl-1-picrylhydrazyl and Hydroxyl radicals scavenging activities, and inhibition of lipid peroxidation potential of the extract were assessed. Furthermore, rats (n=21) randomized into three groups were exposed to 50, 150, and 250 mg/kg body weight of the extract for 30 days. Control animals (n=7) received corn oil, after which blood and liver were excised for antioxidant assay.
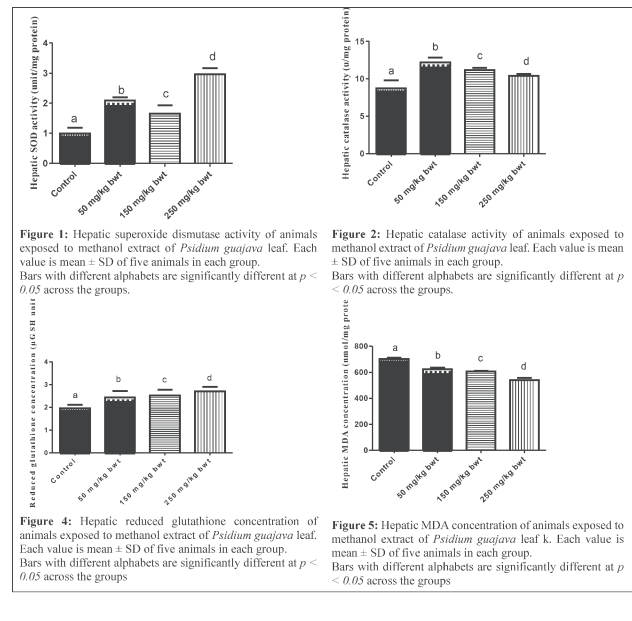
Results: The extract is rich in phenolic and flavonoid compounds. It scavenged DPPH and hydroxyl radicals and inhibits lipid peroxidation in-vitro. In-vivo, it increased the activities of hepatic superoxide dismutase, catalase, glutathione peroxidase, and plasma paraoxonase, and the concentration of hepatic reduced glutathione and MDA.
Conclusion: Psidium guajava leaf extract is a potential source of natural antioxidant compounds, capable of supplementing the body's antioxidant defense system.
References
Kumar M, Saurabh V, Tomar M, Hasan M, Changan S, Sasi M, et al. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021; 10: 299.
Sharma A, del Carmen Flores-Vallejo R,
Cardoso-Taketa A, Villarreal ML. Antibacterial activities of medicinal plants used in Mexican traditional medicine. J. Ethnopharmacol. 2017; 208: 264–329.
Mannino G, Gentile C, Porcu A, Agliassa C, Caradonna F, Bertea CM. Chemical profile and biological activity of cherimoya (Annona cherimola Mill.) and atemoya (Annona atemoya) leaves. Molecules. 2020; 25: 2612.
Ashraf A, Sarfraz RA, Rashid MA, Mahmood A, Shahid M, Noor N. Chemical composition, antioxidant, antitumor, anticancer and cytotoxic effects of Psidium guajava leaf extracts. Pharm.
Biol. 2016; 54: 1971–1981.
Jiang L, Lu J, Qin Y, Jiang W, Wang Y. Antitumor effect of guava leaves on lung cancer: A network pharmacology study. Arab. J. Chem. 2020; 13: 7773–7797.
Farag RS, Abdel-Latif MS, Abd El Baky HH, Tawfeek LS. Phytochemical screening and antioxidant activity of some medicinal plants' crude juices. Biotechnol. Rep. 2020; 28: e00536.
Luca SV, Macovei I, Bujor A, Miron A, SkalickaWo´zniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020; 60: 626–659.
Taha TF, Elakkad HA, Gendy ASH, Abdelkader MAI, Hussein SSE. In vitro bio medical studies on Psidium guajava leaves. Plant Arch. 2019; 19: 199–207.
Mc-Donald S, Prenzler PD, Autolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001; 73: 73.
Chang CC, Yang MH, Wen HM, Chern JC.
Estimation of total Flavonoid content of propolis by two complimentary colorimetric methods. J. Food. Drug. Analysis. 2002; 10: 117-182.
Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001; 15: 127-130.
Aruoma OI, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987; 241(1): 273-278.
Ruberto G, Baratta MT, Deans SG, Dorman HJD. Analysis of chemical composition and bioactive property evaluation. Asian Pac. J. 2020; 31: 3-15.
Arthur JR. Superoxide dismutase and glutathione peroxidase activities in neutrophils from selenium deficient and copper deficient cattle. Life Sci. 1985; 36(16): 1569-1575.
Aebi, H. Catalase. In: Bergmeyer (ed) Methods of enzymatic analysis. 1974. 2nd ed, Vol. 2. Amsterdam: Elsevier, pp. 673–684.
Rotruck JT, Pope AL, Ganther HE, Swanson AB,
Hafeman DG, Hoekstra WG. Selenium:
Biochemical role as a component of glutathione peroxidase. Science. 1973; 179 (4073): 588-590.
Moron, M.S., Depierre, J.W. and Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979. 582: 67–78.
Buege, J.A. and Aust, S.D. Microsomal lipid peroxidation. In: Sidney, F. and Lester, P. (eds) Methods in enzymology. 1978. Amsterdam: Elsevier, pp. 302–310.
Furlong CE, Richter RJ, Seidel SL, Costa LG, Motulsky AG. Spectrophotometric assay for the enzymatic assay for the enzymic hydrolysis of active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Analy Biochem. 1989; 180: 242-247.
Sagar B, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011; 48(4): 412-422.
Sasho G, Rafal S, Stephane B, Davide V. Environmental implications of hydroxy radicals. Chem Rev. 2015; 115(24): 13051-13092.
Lee S, Olga B, Natalia Y. Effect of lipid peroxidation products on the activity of human retinol dehydrogenase 12 (RDH12) and retinoid metabolism. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2008; 1782(6): 421-425.
Ankit T, Raina R, Banga A, Gulati N, Juneja S, Shetty DC. Superoxide Dismutase response: physiological plasticity in tobacco users. Minerva Stomatol. 2019; 68(1): 25-30.
Valko M, Rhodes C, Moncol J, Izakovic MM, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006; 160: 1-40.
Edithlubo L, Kelly NJ, Oldebeken SR, Leopold JA, Zhang YY, Loscalzo J, et al. Glutathione peroxidase-1 deficiency augments
How to cite this article:
proinflammatory cytokine-induced redox. J Biol Chem. 2011; 286(41): 35407-35417.
Katia A, Baldelli S, Ciriolo MR. Glutathione: new roles in redox signaling for an old antioxidant. Front Pharmacol. 2014; 26(5): 196.
Del Rio D, Stewart AJ, Pellegrini N, A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005; 15(4):316-328.
Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005; 46: 1239-1247.
Mackness MI, Arrol S, Abbott C, Durrington PN. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein-associated paraoxonase.
Atherosclerosis. 1993; 104(1-2):129-135.
Aviram M, Rosenblat M, Bisgaier CL, Newton RS, Primo-Parmo SL, La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998; 101: 1581-1590.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

