Ascorbic acid modulates prefrontal cortex cellular changes in androgen deprived rats
Keywords:
Ascorbic acid, Orchiectomy, Prefrontal cortex, Testosterone deficiency, nitrosative and oxidative stressAbstract
Background: Testosterone deficiency has been implicated in numerous neurodegenerative diseases such as Alzheimer's, Parkinson and Huntington's disease. We used a model of androgen deprived rats to determine the effects of ascorbic acid on prefrontal cortex (PFC) cellular changes associated with a subset population of androgen deprived patients.
Methodology: Chemical castration (using testosterone antagonist) as well as orchiectomy can induce androgen deprivation. Twenty-one (21) adult male Wistar rats with an average weight of 170g±10g were randomly assigned into three groups with each group containing seven (7) rats. Group A was control group, Group B= Orchiectomy + Flutamide (11 mg/kg body weight), and group C= Orchiectomy + Flutamide (11 mg/kg body weight)+Ascorbic acid (100 mg/kg body weight). Treatment lasted for 30 days. Nitric oxide and Malondialdehyde levels were assessed; while serum testosterone level was assayed. Histological, Histochemical, and immunohistochemical investigations were performed using Hematoxylin & Eosin, Cresyl fast violet, and Bielschowsky stains respectively.
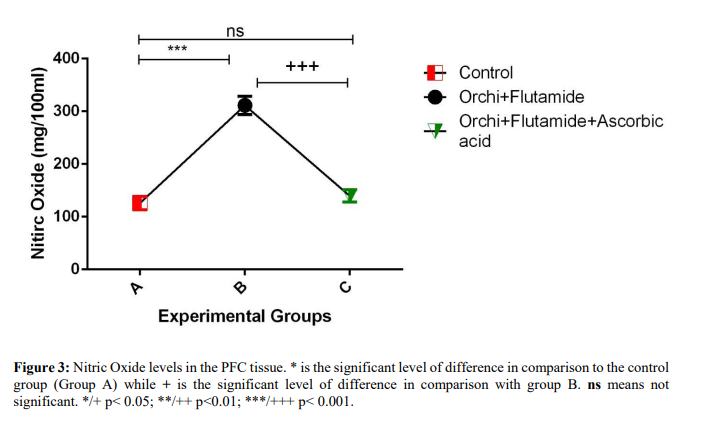
Result: Our results showed increased expression of Nitric Oxide (NO), and increased lipid peroxidation (MDA) in the PFC of orchiectomized animals with altered cytoarchitectural morphology evidenced by decreased Nissl staining polarity in neuronal axons and aggregation of neurofibrillary tangles. Oxidative and nitrosative stress were well modulated in animals treated with ascorbic acid with unaltered prefrontal cortex morphology.
Conclusion: The results indicated that decline in brain androgen activities caused nitrosative and oxidative stress-driven pathology in the prefrontal cortex while supplementing endogenous ascorbic acid offered therapeutic value by scavenging free radicals in the prefrontal cortex
References
Nyby JG. Reflexive testosterone release: a model system for studying the nongenomic effects of testosterone upon male behavior. Frontiers in neuroendocrinology. 2008;29(2):199-210.
Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS)
administration on reproductive and aggressive behaviors in male rats. Behavioral neuroscience. 2003;117(5):904.
Fargo KN, Galbiati M, Foecking EM, Poletti A, Jones KJ. Androgen regulation of axon growth and neurite extension in motoneurons. Hormones and behavior. 2008;53(5):716-28.
Taylor GT, Maloney S, Dearborn J, Weiss J. Hormones in the mentally disturbed brain: steroids and peptides in the development and treatment of psychopathology. Central Nervous
System Agents in Medicinal Chemistry
(Formerly Current Medicinal Chemistry-Central Nervous System Agents). 2009;9(4):331-60.
Eleawa SM, Sakr HF, Hussein AM, Assiri AS, Bayoumy NM, Alkhateeb M. Effect of testosterone replacement therapy on cardiac performance and oxidative stress in orchidectomized rats. Acta physiologica. 2013;209(2):136-47.
Azevedo RB, Lacava ZG, Miyasaka CK, Chaves SB, Curi R. Regulation of antioxidant enzyme activities in male and female rat macrophages by sex steroids. Brazilian Journal of Medical and Biological Research. 2001 May;34(5):683-7.
Mancini A, Leone E, Festa R, Grande G,
Silvestrini A, De Marinis L, et al., Effects of testosterone on antioxidant systems in male secondary hypogonadism. Journal of andrology. 2008;29(6):622-9.
Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Hormones and behavior. 2008;53(5):63846.
Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, et al., Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PloS one. 2012;7(1):e29598.
Akinola OB, Gabriel MO. Neuroanatomical and molecular correlates of cognitive and behavioural outcomes in hypogonadal males. Metabolic brain disease. 2018;33(2):491-505.
Mitra S, Behbahani H, Eriksdotter M. Innovative therapy for Alzheimer's disease-with focus on biodelivery of NGF. Frontiers in neuroscience. 2019 Feb 5;13:38.
Kluger J, Roy A, Chao HH. Androgen deprivation therapy and cognitive function in prostate cancer. Current Oncology Reports. 2020;22(3):24.
Anand-Ivell R, Wohlgemuth J, Haren MT, Hope PJ, Hatzinikolas G, Wittert G, et al., Peripheral INSL3 concentrations decline with age in a large population of Australian men. International journal of andrology. 2006;29(6):618-26.
Bianchi VE, Rizzi L, Bresciani E, Omeljaniuk RJ, Torsello A. Androgen therapy in neurodegenerative diseases. Journal of the Endocrine Society. 2020;4(11):bvaa120.
Bansal R, Singh R. Exploring the potential of natural and synthetic neuroprotective steroids against neurodegenerative disorders: A literature review. Medicinal research reviews.
;38(4):1126-58.
Son SW, Lee JS, Kim HG, Kim DW, Ahn YC, Son CG. Testosterone depletion increases the susceptibility of brain tissue to oxidative damage in a restraint stress mouse model. Journal of neurochemistry. 2016;136(1):106-17.
Toro-Urrego N, Garcia-Segura LM, Echeverria V, Barreto GE. Testosterone protects mitochondrial function and regulates neuroglobin expression in astrocytic cells exposed to glucose deprivation. Frontiers in aging neuroscience. 2016;8:152.
Agostinho P, A Cunha R, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Current pharmaceutical design. 2010;16(25):2766-78.
Anderson J. The role of antiandrogen monotherapy in the treatment of prostate cancer. BJU international. 2003;91(5):455-61.
Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB,
Van Poppel HE, Tammela TL, et al., Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. The Journal of urology. 2000;164(5):1579-82.
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al., Vitamin C as an antioxidant: evaluation of its role in disease prevention. Journal of the American college of Nutrition. 2003;22(1):18-35.
Idris AI. Ovariectomy/orchidectomy in rodents. InBone Research Protocols 2012 (pp. 545-551). Humana Press, Totowa, NJ.
Yawson EO, Akinola OB. Hippocampal cellular changes in androgen deprived insulin resistant rats. Metabolic Brain Disease. 2021;36(5):103748.
Matsumoto AM, Bremner WJ. Serum testosterone assays—accuracy matters. The
Journal of Clinical Endocrinology &
Metabolism. 2004;89(2):520-4.
Buege JA, Aust SD. [30] Microsomal lipid peroxidation. In Methods in enzymology 1978;52:302-310. Academic press.
Nims RW, Darbyshire JF, Saavedra JE, Christodoulou D, Hanbauer I, Cox GW, Grisham MB, Laval F, Cook JA, Krishna MC, Wink DA. Colorimetric methods for the determination of nitric oxide concentration in neutral aqueous solutions. Methods. 1995;7(1):48-54.
Bancroft JD, Layton C. The hematoxylins and eosin. Bancroft's theory and practice of histological techniques. 2012:173-86.
Kádár A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissl staining.
Journal of neuroscience methods.
;184(1):115-8.
Litchfield S, Nagy Z. New temperature modification makes the Bielschowsky silver stain reproducible. Acta neuropathologica. 2001;101(1):17-21.
Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutrition, metabolism and cardiovascular diseases. 2005;15(4):316-28.
Padurariu M, Ciobica A, Lefter R, Lacramioara Serban I, Stefanescu C, Chirita R. The oxidative stress hypothesis in Alzheimer's disease. Psychiatria Danubina. 2013;25(4):0-409.
Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environmental Toxicology: An International Journal. 2009;24(1):66-73.
Rosa SD, Cirillo P, Paglia A, Sasso L, Palma VD, Chiariello M. Reactive oxygen species and antioxidants in the pathophysiology of cardiovascular disease: does the actual knowledge justify a clinical approach? Current vascular pharmacology. 2010;8(2):259-75.
Abayomi TA, Tokunbo OS, Adebisi BT, Fadare MU, Gbadamosi IT, Akinwale JO, et al.,
Neurobehavioral assessment of the impact of
How to cite this article:
vitamins C and E following acute exposure to sodium azide-induced neurotoxicity. Journal of Environmental Toxicology and Public Health. 2019;4:15-20.
Avontuur JA, Bruining HA, Ince C. Sepsis and nitric oxide. In Oxygen Transport to Tissue XVII 1996 (pp. 551-567). Springer, Boston, MA.
Deyhim F, Lopez E, Gonzalez J, Garcia M, Patil BS. Citrus juice modulates antioxidant enzymes and lipid profiles in orchidectomized rats. Journal of medicinal food. 2006;9(3):422-6.
Beck KF, Eberhardt W, Walpen S, Apel M, Pfeilschifter J. Potentiation of nitric oxide synthase expression by superoxide in interleukin 1β-stimulated rat mesangial cells. FEBS letters. 1998;435(1):35-8.
Traish A, Bolanos J, Nair S, Saad F, Morgentaler
A . D o a n d r o g e n s m o d u l a t e t h e pathophysiological pathways of inflammation? Appraising the contemporary evidence. Journal of clinical medicine. 2018;7(12):549.
May JM. How does ascorbic acid prevent endothelial dysfunction?. Free radical biology and medicine. 2000;28(9):1421-9.
Solerte SB, Fioravanti M, Schifino N, Cuzzoni G,
F o n t a n a I , Vi g n a t i G , e t a l . , .
Dehydroepiandrosterone sulfate decreases the interleukin-2-mediated overactivity of the natural killer cell compartment in senile dementia of the Alzheimer type. Dementia and geriatric cognitive disorders. 1999;10(1):21-7.
Hsieh HL, Yang CM. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed research international. 2013;2013-2226.
Hazewindus M, Haenen GR, Weseler AR, Bast A. The anti-inflammatory effect of lycopene complements the antioxidant action of ascorbic acid and α-tocopherol. Food Chemistry. 2012;132(2):954-8.
Solerte SB, Fioravanti M, Schifino N, Cuzzoni G,
Fontana I, Vignati G, et al., .
Dehydroepiandrosterone sulfate decreases the interleukin-2-mediated overactivity of the natural killer cell compartment in senile dementia of the Alzheimer type. Dementia and geriatric cognitive disorders. 1999;10(1):21-7.
Jahić A, Žnidarič MT, Pintar S, Berbić S, Žerovnik E. The effect of three polyphenols and some other antioxidant substances on amyloid fibril formation by Human cystatin C.
N e u r o c h e m i s t r y i n t e r n a t i o n a l .
;140:104806.
Barten DM, Albright CF. Therapeutic strategies for Alzheimer's disease. Molecular neurobiology. 2008;37(2):171-86.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

