Response to neoadjuvant chemotherapy in breast cancer in a resource limited environment.
Keywords:
Neoadjuvant chemotherapy, breast cancer, clinical response, compliance, limited resourceAbstract
Objective: Assess the outcome of neoadjuvant chemotherapy using Adriamycin and Cyclophosphamide followed by Paclitaxel (AC-P regime) in breast cancer.
Methods: A prospective observational study of newly diagnosed breast cancer patients with palpable breast lumps on neoadjuvant chemotherapy of AC-P regime. Age of the patients, tumour size, stage, estrogen, progestogen and HER2 receptor status were noted. Tumour size measured at presentation, first, third, fifth, sixth and eighth doses to determine response as defined by the UICC method i.e. complete clinical response, partial clinical response, stable disease and progressive disease.
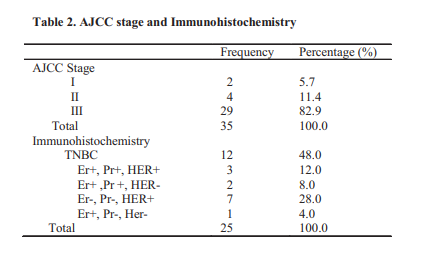
Results: Complete clinical response was observed in 40% of 35 patients studied. Complete clinical response was found in 81.8% tumours less than 5cm in diameter while 20.8% of tumours greater 5cm.had complete clinical response. (X2 =11.6, p= 0.001) Eighty-eight percent complied with treatment schedule. Mastectomy was done in 34.2%, Breast conservation surgery (BCS) in 14.2%, and 17.1% lost to follow up.
Conclusion: Neoadjuvant chemotherapy using AC-P sequential regime is effective in breast cancer with tolerable side effects and excellent treatment compliance in the study population.
References
Anderson BOJ, Yip CH, Smith RA, et al Guidelines implementation for breast health care in low-income and middle-income countries. Overview of Breast Health Global Initiative Global Summit 2007. Cancer 2008. 113:2221-43
Adebamowo CA, Ajayi OO, Breast Cancer in Nigeria. West Afr J Med 2000;19:179-91 3. Ayoade BA, Agboola AJ, Olatunji AA, et al. Clinical characteristics and survival outcome of Breast Cancer in Southwest Nigerian women. J. Afr. Cancer 2014; 6:79-84. DOI 10.1007/s12558-
-0311-8
Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective,
comparative, interventional cohort study of 13722 women. BMJ 2012;344:e2718 5. Gralow JR, Burstein HJ, Wood W, et al
.Preoperative therapy in invasive breast cancer: pathologic assessment and systemic issues. J Clin Oncol 2008; 26:814
Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J.Clin Oncol 2006;24:1940
Schwartz GF, Hortobagyi GN. Proceedings of consensus conference on neoadjuvant
chemotherapy in carcinoma of the breast, April 26-28 2003, Philadephia, Pennsylvania. Cancer 2004; 100:2512
[Guideline] NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. Version1.2017.
National Comprehensive Cancer Network.
A v a i l a b l e a t
http:/www.ncn.org/professional/physician_gls/p df/breast.pdf. March 10; Accessed on April 14.2017
World Health Organization. National cancer control programmes. Geneva: World Health Organization; 2002.
Sobin LH, Wittekind CH, editors. UICC: TNM classification of malignant tumours.6thed.New York: Wiley-Liss; 2002
Sparano JA, Wang M, Martino S, et al. Weekly Paclitaxel in the adjuvant treatment of Breast cancer. N Eng J Med 2008: 358: 1663
Hayward JL, Carbone PP, Heuson JC, Kumaoka S, Segaloff A, Rubens RD,. Assesment of response to therapy in advanced breast cancer: a project of the Programme on Clinical Oncology of the International Union Against Cancer, Geneva Switzerland. Cancer 1977; 39: 1289-94
Oluwole SF, Fadiran OA, Odesanmi WO.
Diseases of the breast in Nigeria. Br J Surg 1987; 74: 582-5
Conflict of interest: The authors declare no Pathological response after two cycles of
Awad Ali. M .A. Evaluation of clinical and neoadjuvant chemotherapy on Sudanese patients with locally advanced breast cancer. Ethiop J Health Sci. 2014 ;24(1) 16-20
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine -year result from National adjuvant breast and bowel project B-18.
Journal of the National Cancer Institute Monograph 2001;30:96-102
Arowolo OA, Akinkuolie AA, Lawal OO, Alatise OI, Salako AA, Adisa AO. The impact of
Neoadjuvant Chemotherapy on Patients with Locally advanced Breast Cancer in a Nigerian
Semi urban Teaching Hospital: A single center
Descriptive study. World J Surg. 2010; 34: 1771-
Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E and Wolmark N. Pathology of preoperative chemotherapy: Findings from National Surgical Adjuvant Breast and Bowel (NASBP) protocol B-
Cancer , 2002;95:681-695
Honkoop AH, Lukx-de Bakker SA, Hoekman K eet al. Prolonged neoadjuvant chemotherapy with GM-CSF in locally advanced breast cancer. Oncologist, 1999; 4: 106-111
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, Brown Am, et al Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer. National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2006;24(13): 2019 -2027
Chollet P, Charrier S, Brian E et al. Clinical and pathological response to primary neoadjuvant chemotherapy in operable breast cancer. Eur J Cancer, 1997; 33: 862-866
Kaufman M, Von Minckwitz G, Bear HD, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol 2007; 18(12): 1927-1934
Untch M, von Minckwitz. Neoadjuvant Chemotherapy: Early response as a Guide for further treatment: Clinical, Radiological and biological. J Natl Cancer InstMonogr 2011;43:138-141
Tewari M, Krishnamurthy A, Shukla HS. Predictive markers of response to neoadjuvant chemotherapy in Breast cancer. Surgical Oncology 2008; 17:301-311
HuoberJ, von Minckwitz G, Denkert C, et al: Effect of neoadjuvant anthracycline-taxane based chemotherapy in different biological breast cancer phenotypes: overall results from the GeparTrio study. Breast Cancer Res Treat 2010; 124: 133-140
Loibl S, Jackish C, Gade S, et al Neoadjuvant chemotherapy in the very young 35 years of age or younger. Cancer Res 2012;72 (suppl24):S3-1
Bonnadonna G, Valgussa P, Zucali R, SalvadoriB. Primary chemotherapy in surgically resectable breast cancer. CA; A cancer Journal for clinicians ; 45: 227-43
Esserman LJ, Berry DA, Cheang MC, et al. Chemotherapy response and recurrence- free survival in neoadjuvant breast cancer depends on biomarker profiles: results from I-SPY1 Trial (CALGB 150007/150012). Breast Cancer Res Treat 2012;132: 1049-1062
Clegg-Lamptey J, Hodasi W. A study of breast cancer in Korle Bu Teaching Hospital: Assessing the impact of health education. Ghana Med J 2007;41: 72-7
Fisher B, Brown AM, Dimitrov NV, Poisson R, Redmond C, Margolese RG, et.al, Two months of doxorubicin-cyclophosphamide with and without interval reinduction therapy compared with 6months of cyclophosphamide, methotrexate and fluorouracil in positive-node breast cancer patients with tamoxifen- nonresponsive tumours: Results from the National Surgical Adjuvant Breast and Bowel Project B-15. J Clin Oncol 1990;8:1483-96
Egwuonwu OA, Anyanwu SNC, Nwofor AME. Default from Neoadjuvant chemotherapy in premenopausal female breast cancer patients: What is to blame? Niger Jour Clin Pr. 2012;15:265-269
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

