Moringa oleifera attenuates biochemical and histological changes associated with the pancreas in nicotine-treated rats
Keywords:
Nicotine, Pancreatic damage, Moringa oleiferaAbstract
Objective: The study was undertaken in order to evaluate the beneficial potential of Moringa oleifera, in nicotine-induced pancreatic injury.
Method: Forty-five adult female albino rats were divided into 5 groups A-E, each group having nine rats.
Group A received normal saline; group B received 6.88 mg/kg of nicotine intraperitoneally (i.p); group C received 6.88 mg/kg of nicotine i.p. and 200 mg/kg of Moringa oleifera leaf powder dissolved in 2 ml of normal saline (orally); group D received 13.76 mg/kg of nicotine i.p., while group E received 13.76 mg/kg of nicotine i.p. and 200 mg/kg of Moringa oleifera leaf powder dissolved in 2 ml of normal saline (orally). Treatment was for 8 days and the rats were sacrificed after 24 hours of termination of study. Intracardial blood specimens were obtained to analyse blood glucose, while the pancreas was excised and either fixed in 4% paraformaldehyde for histology or sucrose solution and homogenised for biochemical analysis of lactate dehydrogenase (LDH) and glucose-6-phosphate dehydrogenase (G-6-PDH) enzymes.
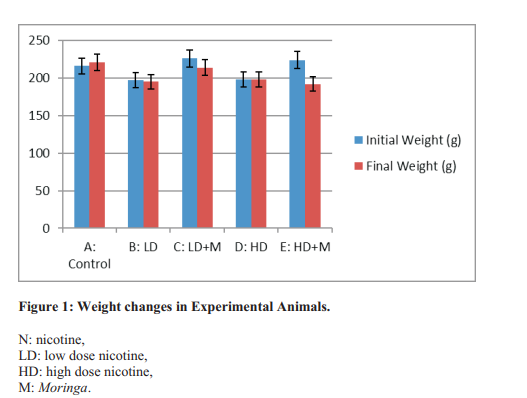
Results: In comparison with the Control, animals treated with low dose of nicotine with or without Moringa oleifera and those treated with high dose of nicotine plus Moringa oleifera had reduction in body weights (p>0.05), while marked reduction in pancreatic weights was noted in low dose nicotine (p<0.05) and both nicotine groups co-treated with Moringa oleifera (p<0.05). There were no significant changes in the levels of blood glucose and pancreatic G-6-PDH levels, while significant reduction occurred in pancreatic LDH levels in nicotine-treated rats (p<0.05). However, LDH improved following coadministration with Moringa oleifera. Observation of the histology of the pancreas revealed atrophy of intercalated ducts, poorly delineated and disintegrating islet of Langerhans in animals treated with the higher dose of nicotine, while changes in pancreatic tissue in animals co-treated with Moringa oleifera were not as severe as the nicotine-treated animals.
Conclusion: Moringa oleifera leaf decoction minimally ameliorates morphological and biochemical changes associated with nicotine-induced pancreatic damage.
References
Benowitz NL. Nicotine addiction. N Engl J Med, 2010; 362:2295-2303.
Lombardi EMS, Prado GF, Santos UP, Fernandes FLA. Women and smoking: risks, impacts, and challenges. J. Bras. Pneumol. 2011; 37(1):118128.
Mackay J, Eriksen M. The tobacco atlas. Geneva: World Health Organization, 2002.
Gamieldien K, Maritz GS. Postnatal expression of cytochrome P450 1A1, 2A3 and 2B1 in neonatal rat lung: influence of maternal nicotine exposure. Exp Lung Res 2004; 30:121-133
Pool WF, Godin CS, Crooks PA. Nicotine racemization during nicotine smoking. The Toxicologist 1985; 5:232.
Imoto M, DiMagno EP. Cigarette smoking increases the risk of pancreatic calcification in late-onset but not early-onset idiopathic chronic pancreatitis. Pancreas. 2000; 21:115-119.
Morton C, Klatsky AL, Udaltsova N. Smoking, coffee, and pancreatitis. Am J Gastroenterol. 2004; 99:731-738.
Edderkaoui M, Thrower E. Smoking and pancreatic disease. J Cancer Ther 2013; 4(10A):34-40.
Chowdhury P, Rayford PL. Smoking and pancreatic disorders. Eur J Gastroenterol. Hepatol. 2000; 12:869–877.
van Westerloo DJ, Giebelen IA, Florquin S, Bruno MJ, Larosa GJ, Ulloa L. et al. The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice.
Gastroenterology. 2006; 130(6):1822-30.
Bruin JE, Kellenberger, LD, Gerstein HC, Morrison KM, Holloway AC. Foetal and neonatal nicotine exposure and postnatal glucose homeostasis: identifying critical windows of exposure. J Endocrinol. 2007; 194:171-178.
Somm E, Schwitzgebel V, Vauthay D, Camm E, Chen C, Giacobino J. et al. Prenatal nicotine exposure alters early pancreatic islet and adipose tissue development with consequences and the control of body weight and glucose metabolism later in life. Endocrinology 2008; 149: 6289-99.
Somm E, Schwitzgebel V, Vauthay D, Aubert M, Huppi P. Prenatal nicotine exposure and the programming of metabolic and cardiovascular disorders. Mol. Cell. Endocrinol. 2009; 304:69–77.
Fahey JW. Moringa oleifera: A Review of the
Medical Evidence for Its Nutritional,
Therapeutic, and Prophylactic Properties. Part 1.
Tree of life Journal.2005;1:5. URL:
http://www.tfljournal.org/article.php/200512011 24931586. Accessed on 15/10/2016.
Promkum C , Kupradinun P, Tuntipopipat S,
Butryee C. Nutritive evaluation and effect of Moringa oleifera pod on clastogenic potential in the mouse. Asian Pac. J. Cancer Prev. 2010; 11(3):627-632
Tsaknis J, Lalas S, Gergis V, Dourtoglou V, Spiliotis V. Characterization of Moringa oleifera variety Mbololo seed oil of Kenya. J Agric Food Chem. 1999; 47(11):4495-9.
Rao AV, Devi PU, Kamath R. In vivo radioprotective effect of Moringa oleifera leaves. Indian Journal of Experimental Biology 2001;39:858-63
Hamza AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food Chem Toxicol. 2010; 48(1):345-55.
Sathya TN, Aadarsh P, Deepa V, Murthy PB. Moringa oleifera Lam. leaves prevent cyclophosphamide-induced micronucleus and DNA damage in mice. International Journal of Phytomedicine 2010; 2(2): 147-154.
Sreelatha S, Jeyachitra A, Padma PR.
Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cells. Food Chem Toxicol. 2011;49:1270–1275.
Sharma V, Paliwal R. Chemo Protective role of Moringa oleifera and its isolated Saponin against DMBA induced Tissue Damage in male mice: A Histopathological analysis. Int. J. Drug Dev. & Res. 2012; 4(4): 215-228.
Omotoso GO, Gbadamosi IT, Afolabi TT, Abdulwahab AB, Akinlolu AA. Ameliorative effects of Moringa on cuprizone-induced memory decline in rat model of multiple sclerosis. Anat Cell Biol. 2018; 51:119-127.
Saini RK, Sivanesan I, Keum Y-S.
Phytochemicals of Moringa oleifera: a review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6(2):203. doi:10.1007/s13205-016-0526-3.
Omotoso GO, Gbadamosi IT, Olajide OJ, DadaHabeeb SO, Arogundade TT, Yawson EO. Moringa oleifera phytochemicals protect the brain against experimental nicotine-induced neurobehavioural disturbances and cerebellar degeneration. Pathophysiology, 2018; 25: 57–62.
Nweze NO, Nwafor FI. Phytochemical, proximate and mineral composition of leaf extracts of Moringa oleifera Lam. from Nsukka, South-Eastern Nigeria. J. Pharm. Biol. Sci. 2014; 9: 99–103.
Brèiæ KI. Facts about nicotine toxicity. Arh Hig Rada Toksikol (Archives of Industrial Hygiene and Toxicology) 2005; 56:363-371.
Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969; 6:24-27.
Cheesebrough M. District Laboratory practice in tropical countries Part 1. Cambridge: Cambridge University Press, 2005.
Weisshaar D, Gossrau E, Faderl B. Normal ranges of alpha-HBDH, LDH, AP, and LAP as measured with substrate-optimated test charges. Med Welt. 1975; 26(9):387-92.
Lohr GW, Waller HD. Glucose-6-phosphate dehydrogenase methods of enzymatic analysis, 3rd Ed., Wehnheim: Varlag Chemie, 1974.
Pearse EAG. Histochemistry: Theoretical and Applied, 4th ed. Vol. 1 and 2. Edinburgh, Churchill Livingstone. 1980.
John U, Hanke M, Rumpf H-J, Thyrian JR. Smoking status, cigarettes per day, and their relationship to overweight and obesity among former and current smokers in a national adult general population sample. Int J Obes 2005; 29:1289–1294.
Chiolero A, Faeh D, Paccaud F, Cornuz J . Consequences of smoking for body weight, body fat distribution, and insulin resistance. The American Journal of Clinical Nutrition, 2008; 87(4): 801–809
Bamia C, Trichopoulou A, Lenas D, Trichopoulos D. Tobacco smoking in relation to body fat mass and distribution in a general population sample. Int J Obes Relat Metab Disord. 2004; 28(8):10916.
Frati AC, Iniestra F, Ariza CR. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care 1996; 19(2): 112-118.
Bajaj M. Nicotine and insulin resistance: when the smoke clears. Diabetes 2012; 61(12): 30783080.
Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall JE, Landau LI et al. Perinatal and childhood origins of cardiovascular disease. Int J Obes 2007; 31:236–244.
Bornemisza P, Suciu I. Effect of cigarette smoking on the blood glucose level in normals and diabetics. Med Interne. 1980; 18(4):353-6.
Bruin JE, Gerstein HC, Morrison KM, Holloway AC. Increased pancreatic beta-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicol Sci. 2008; 103:362–70.
Zimmerman M, McGeachie J. The effect of nicotine on aortic endothelium: A comparative study. Atherosclerosis 1987; 63:33-41.
Aoshiba K, Nagai A, Ysui S, Konno K. Nicotine prolongs neutrophil survival by suppression of apoptosis. J Lab Clin Med.1996; 127:186–194.
Bais S, Singh GS, Sharma R. Antiobesity and hypolipidemic activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Advances in Biology, 2014; Article ID 162914. https://doi.org/10.1155/2014/162914.
Ezeigbo O R, Barrah CS, Ezeigbo IC.
Phytochemical analysis and antidiabetic effect of aqueous and ethanolic extracts of Moringa oleifera leaves in alloxan-induced diabetic Wistar albino rats using insulin as reference drug. International Journal of Diabetes Research, 2016; 5(3): 48-53
Khan W, Parveen R, Chester K, Parveen S, Ahmad S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GCMS metabolomics. Frontiers in Pharmacology. 2017;8:577. doi:10.3389/fphar.2017.00577. 45. Villarruel-López A, López-de la Mora DA,
Vázquez-Paulino OD, Puebla-Mora AG, TorresVitela MR, Guerrero-Quiroz LA et al. Effect of Moringa oleifera consumption on diabetic rats.
BMC Complement Altern Med. (2018) 18:127.
https://doi.org/10.1186/s12906-018-2180-2
Panda S. Butanolic fraction of Moringa oleifera Lam. (Moringaceae) attenuates isoprotrenolinduced cardiac necrosis and oxidative stress in rats: an EPR study. EXCLI Journal. 2015;14:6474.
Pinna A, Contini EL, Carru C, Solinas G. Glucose-6-phosphate dehydrogenase deficiency and diabetes Mellitus with severe retinal complications in a Sardinian population, Italy. Int J Med Sci. 2013; 10(13): 1907–1913.
Gumustekin K, Ciftci M, Coban A, Altikat S, Aktas O, Gul M et al. Effects of nicotine and vitamin E on glucose 6-phosphate
dehydrogenase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem. 2005; 20(5):497-502.
Al-Asmari AK, Albalawi SM, Athar MT, Khan AQ, Al-Shahrani H, Islam M. Moringa oleifera as an anti-cancer agent against breast and colorectal cancer cell lines. PLoS One 2015;
0 ( 8 ) : e 0 1 3 5 8 1 4 . d o i :
1371/journal.pone.0135814.
Berkovich L, Earon G, Ron I, Rimmon A, Vexler A, Lev-Ari S. Moringa oleifera aqueous leaf extract down-regulates nuclear factor-kappaB and increases cytotoxic effect of chemotherapy in pancreatic cancer cells. BMC Complement Altern Med. 2013;13:212.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

