Morphometric variables of offspring of Quassia amara treated male rats
Keywords:
Quassia amara, spermatozoa, developmental programming, anogenital distance, endocrine disruptorsAbstract
Objective: Quassia amara is a medicinal plant with various pharmacological properties. The bioactive compound quassin is used as flavoring in food and beverages. Reproductive toxicological action of Q. amara is well documented but no information exists on its effect on prenatal programming.
Methods: Adult male rats (180-200g, n=5) were treated daily (p.o) with Q. amara extract (100 mg/kg) for 6 weeks. A control group received distilled water. After 5 weeks of treatment, female rats were cohabited with the males for 7 days, at the ratio of 2:1. Mating was confirmed by presence of spermatozoa in all vaginal smear. Morphometric indices of all offspring were recorded on postnatal day one. They were also examined for any sign of abnormality or physical defect.
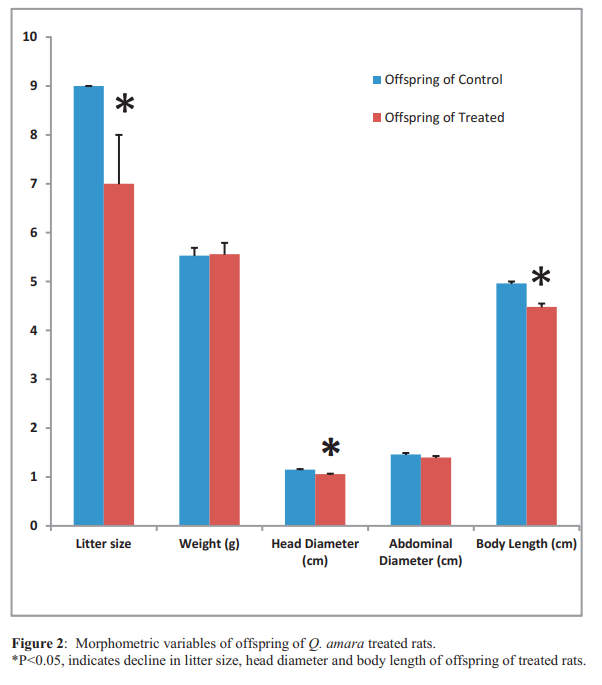
Results: Fertility was zero in four out of the five treated rats. The females that cohabited with the fertile treated male gave birth to pups of varying sizes (6 and 9). However, four of the five control male rats were fertile and the female rats they mated had 9 pups each. No visible physical defect was observed on all offspring. Anogenital distance of the male offspring of the treated rats was significantly shorter than male offspring of the control, while anogenital distance of female offspring showed no statistical difference. Head diameter and body length were also significantly lower in offspring of the treated rats. However, weight, abdominal diameter and sex ratio of offspring were not statistically different.
Conclusion: Quassia amara extract caused a reduction in the male anogenital distance, litter size, head diameter and body length of the offspring of treated male rats. Its reproductive toxicity actions may alter the developmental programming and probably transferred from one generation to another.
References
Carlsen E, Giwercman A, Keiding N,
Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992; 305: 609–613.
Eisenberg ML, Hsieh MH, Walters RC, Krasnow R, Lipshultz LI. The Relationship between Anogenital Distance, Fatherhood, and Fertility in Adult Men. PLoS ONE 2011; 6(5): e18973. doi:10.1371/journal.pone.0018973.
Hsieh MH, Breyer BN, Eisenberg ML, Baskin LS. Associations among hypospadias, cryptorchidism, anogenital distance, and endocrine disruption. Curr Urol Rep 2008; 9:
–142.
Thankamony A, Ong KK, Dunger DB, Acerini
CL, Hughes IA. Anogenital distance from birth to 2 years: a population study. Environ Health Perspect 2009; 117: 1786–1790.
Sathyanarayana S, Beard L, Zhou C, Grady R. Measurement and correlates of ano-genital distance in healthy, newborn infants. Int J Androl 2010; 33: 317–323.
Eisenberg ML and Lipshultz LI. Anogenital distance as a measure of human male fertility J Assist Reprod Genet. 2015; 32(3): 479 – 484.
Raji Y and Bolarinwa AF. Antifertility activity of Quassia amara in male rats in vivo study. Life Sciences 1997; 61: 1067-1074.
Obembe OO and Raji Y. Reproductive toxicity of Quassia amara extract: action on sperm capacitation and acrosome reaction. Acad. J. Plant Sci. 2012; 5 (3): 60-69.
Barker DJP. Programming the baby. In: Mothers, babies, and disease in later life. London: BMJ Publishing Group 1994; 14–36.
Drake AJ and Walker BR. Intergenerational consequences of fetal programming: nongenomic mechanism of the interaction of low birth weight and cardiovascular risk. J Endocrinol 2004; 180: 1–16.
Kavlock RJ, Daston GP, DeRosa C, Fenner-Crisp P, Gray LE, Kaattari S et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the
U.S. EPA-sponsored workshop. Environ. Health Perspect 1996; 104: 715–740.
Knez J. Endocrine-disrupting chemicals and male reproductive health. Reproductive BioMedicine Online 2013; 26, 440– 448.
Christiansen S, Axelstad M, Boberg J, Vinggaard AM, Pedersen GA, and Hass U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2014; 147: 477-487.
Jeng HA. Exposure to Endocrine Disrupting Chemicals and Male Reproductive Health. Front
Public Health. 2014; 2: 55: doi: 10.3389/fpubh.2014.00055 PMCID: PMC4046332.
World Medical Association, American
Physiological Society. Guiding principles for research involving animals and human beings. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002; 283: R281-R283.
Ellerman DA, Veronica SB, Silvina PM, Cohen DJ, Conesa D and Cuasnicu PS. Potential Contraceptive Use of Epididymal Proteins: Immunization of Male Rats with Epididymal Protein DE Inhibits Sperm Fusion Ability1. Biology of Reproduction 1998; 59, 1029–1036.
Ostby JS and Gray LE. Transgenerational
(InUtero/Lactational) exposure to investigate the effects of endocrine disrupting compounds (EDCs) in rats. Current Protocols in Toxicology. John Wiley & Sons, Inc 2004. p 16.8.1-16.8.16.
Snedecor GW and Cochran WG. Statistical Methods 7th Ed. Ames. Iowa State University Press 1980. p 215.
Obembe OO, Olaopade JO and Raji Y.
Implication of HongrES1 protein in Quassininduced male reproductive abnormality. Endocrinology and Metabolic Syndrome. 2014; 3 (2): 128. doi:10.4172/2161-1017.1000128.
Fisher SJ. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome.
Reproduction 2004; 127:304-315.
Swan SH, Main KM, Liu F, Stewart SL, Kruse RL et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005; 113: 1056–1061.
Brucker-Davis F, Wagner-Mahler K, Delattre I,
Ducot B, Ferrari P, Bongain A et al.
Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum Reprod 2008;
(8):1708–1810.1093/humrep/den186.
McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls
and risk of hypospadias and cryptorchidism in male offspring. Environ Health Perspect 2009; 117(9):1472–610.1289/ehp.0800389.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

