Alcohol-Diazepam Combination: Implication on the Histoarchitectural Profile of the Hippocampus in Male Rats.
Keywords:
Addiction, abuse, use, dependence, drugAbstract
Objective: Damage to hippocampal neurons has not been described as a constant finding in studies addressing the effects of alcohol and diazepam in laboratory based studies, the present study aimed to elucidating the changes in the histoarchitectural changes of the hippocampus following separate and combined diazepam and alcohol administration in male Wistar rats.
Methodology: Twenty male Wistar rats were randomly divided into four groups (n=5): control (A), 3 mg/kg diazepam (B), 30% v/v ethanol (C), and 30% v/v ethanol plus 3 mg/kg diazepam (D). All administration was done orally for 30 days.
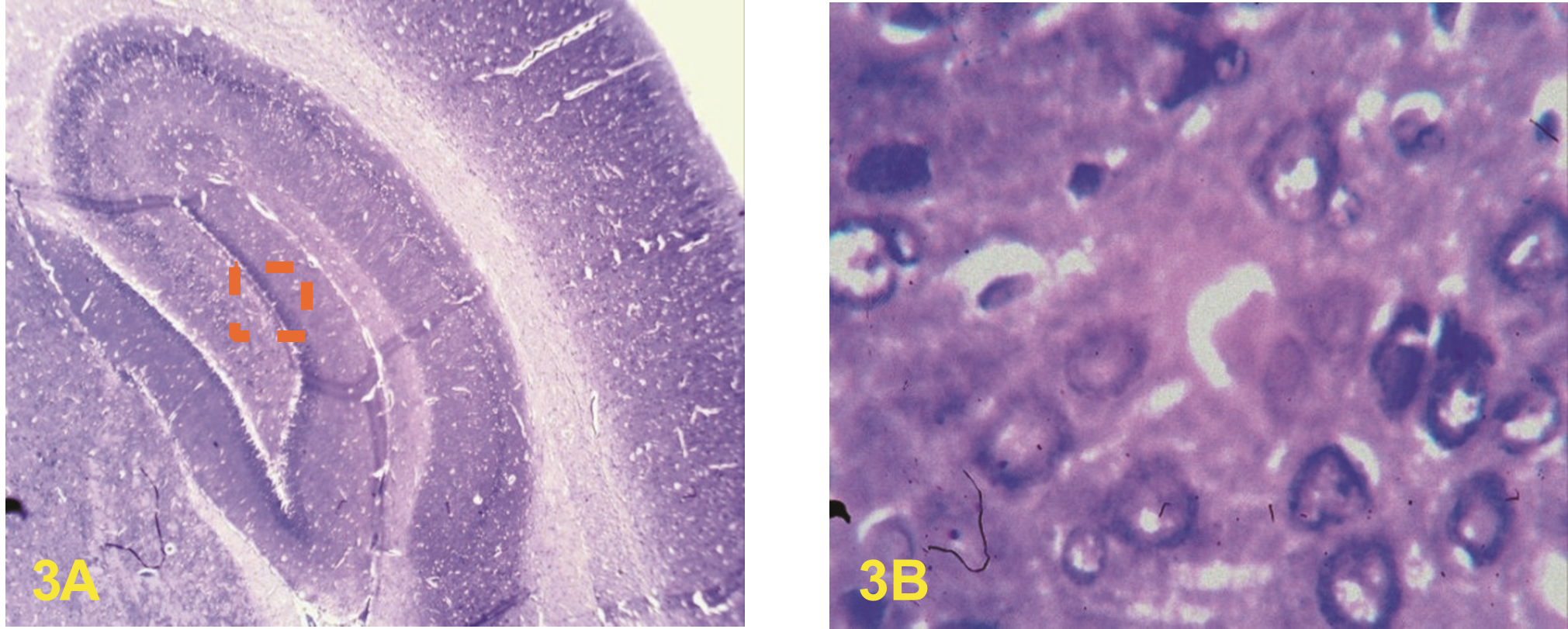
Major findings: There were no distortions in the hippocampal profile of the rats in the control group. The histoarchitectural profile of the hippocampus of the rats in group B showed less significant damage. The histoarchitectural profile of the hippocampus of the rats in group C was disrupted with evidence of enlarged cell bodies and distorted membrane. The hippocampal profile of the rats in group D were with marked neuronal necrosis and variable neuronal loss within the hippocampal subfield.
Conclusion: It was observed that co-administration of ethanol and diazepam conferred neurodegenerative effects on the neuronal profile of the hippocampus in rats.
References
Slavana D, Natasa S, Ljubica G and Varagic V. Behavioural and Endocrine responses of socially isolated rats to long-term diazepam treatment. Acta Veterinaria
(Beograd) 2007. 57(4): 291-302.
Murray JB. Effects of valium and librium on human psychomotor and cognitive functions. Genet Psychol Monogr., 1984. 109 (2): 167-97
Mosallanejad B, Avizeh R, Najafzadeh VH, Pourmedhi M. Evaluation of prophylactic and therapeutic effects of silymarin on mebendazole-induced hepatotoxicity in cats. Comp. Clin Pathol., 2011.10: 1157-64
Maddison JE, Page SW, Church DB.
S m a l l A n i m a l C l i n i c a l
Pharmacology. Elsevier Health
Sciences, 2002; p. 575
Mitra NK and Nagaraja HS. Effect of chronic ethanol exposure on the count of cerebellar Purkinje cells and motor coordination in adult mice. Eur J Anat, 2008. 12 (2): 67-71.
Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res, 2004. 1007: 10-18.
Marlene O and Ksenija M.
Alcoholism and the Brain: An Overview. Alcohol and Health Res., 2003. 27(2):125-133.
Alderazi Y and Brett F. Alcohol and the nervous system. Curr. Diag. Pathol., 2007. 13(3): 203-209.
Johnsen-Soriano S, Bosch-Morell F, Miranda M, Asensio S, Barcia JM, Roma J, et. al., Ebselen prevents chronic alcohol-induced rat hippocampal stress and functional impairment. Alcohol Clin. Exp. Res., 2007. 31(3): 486-92
Chin VS, Van SCE, Matthews DB. Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol. 2010. 44(1): 3-4
Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas
CA3 and CA1. Science. 2004. 305:1295-98.
Samsonovich AV and Nadel L. Fundamental principles and mechanisms of the conscious self. Cortex. 2005. 41(5): 669-89 13. Tracy AL, Jarrard LE, Davidson TL. The hippocampus and motivation revisited: appetite and activity.
Behav. Brain Res., 2001. 127: 13-23.
Garcia-Moreno LM, Santin LJ, Rubio S, Gonzalez-Pardo H, Arias JL. Effects of ethanol and diazepam on AgNOR neuronal activity in the medial mammilary nucleus.
Psicotherma. 1993. 5(1): 125-134.
Kalynchuk LE and Beck CHM. Behavioral analysis of diazepaminduced memory deficits: evidence for sedation-like effects.
Psychopharmacol., 1992. 106: 297302.
Nixon K and Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci., 2004.
(43):9714-22.
Shikanai H, Izumi T, Matsumoto M,
Togashi H, Yamaguchi T, Yoshida T, Yoshioka M. Diazepam-induced increases of synaptic efficacy in the hippocampal-medial prefrontal
cortex pathway are associated with its anxiolytic-like effect in rats. J. Pharmacol. Sci., 2010. 114(3): 341-6
Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008. 42(8): 649-656
Stranahan AM, Jiam NT, Spiegel
AM, and Gallagher M. Aging
Reduces Total Neuron Number in the Dorsal Component of the Rodent Prefrontal Cortex. The J. Comp. Neurol. Res in Sys. Neurosci. 2012. 520:1318–1326
Muhammed OA, Adekomi DA, Enaibe BU and Ademosun AA. Histological, histochemical and immunohistochemical evaluation of the effects of seed and pulp of Carica papaya on the visual relay centres in animal model. J. Med. Plants Res., 2013. 7(16); 1030-1038.
Avwioro OG. Histochemistry and Tissue Pathology: Principles and techniques. 2002. 1st ed,
Claverianum Centre.
Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regal Toxicol. pharmacol., 2000. 32:56-67
Kakkar P, Musavi S, Mehrotra S. Mitochondrial responses under chemical stress. Polish J. Env. Stud., 2000. 9(4): 285-290.
Musavi S and Kakkar P. Effect of diazepam treatment and its withdrawal on pro/antioxidative processes in rat brain. Mol. Cell Biochem., 2003. 245(1-2):51-56.
Kumar S, Dhankhar N, Goyal S, Kar V, Shrivastava M. Hippocampal Injury at Mitochondrial Level Provoked by Iminodipropionitrile, Neuroprotective Effect of Alpha Lipoic Acid. Int. J. Res. in Pharm and Sci. 2011. 1(2): 67-76
Farber JL, Chein KR, Mittnacht S. The pathogenesis of Irreversible cell injury in ischemia; Amer. J. Pathol. 1981. 102:271-281
Felinska W, Pyka U, Szkilnik R, Czechowicz K, Siekierska E, Pilsniak U, Brus R. Effect of ethanol and diazepam given to pregnant rats on the behavior and catecholamine content in the brain of offspring. Pol. J. Pharmacol. Pharm., 1989.41(3):223-5.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

