Cyanide cytotoxicity and the follicular cells of thyroid gland in male Wistar rats
Keywords:
Cyanide, cytotoxicity, cassava, thyroid glandAbstract
Aim: This study was aimed at understanding the cytotoxic effect of cyanide as a result of ingesting inadequately processed cassava products linked with goitre formation as seen in cassava endemic region of Nigeria through cyanide-induced cytotoxicity in rats thyroid gland.
Materials and Methods: Twenty-one F1 Male Wistar rats were randomly grouped into three of seven rats each. The treatment groups (1 & 2), were administered with different concentration of potassium cyanide, while group 3, the control group of the experiment was administered 0.25M sucrose for 30 days. On sacrifice, the rats were bled from which serum FT3, FT4 and TSH concentration were analysed. The excised thyroid gland was processed for light microscopic investigation while the activities of G6PDH, LDH, ALP, MDA and SOD were assayed from the thyroid tissue homogenates.
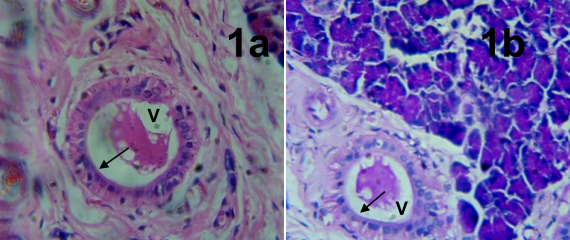
Results: Histological observation of thyroid gland of rats from the experimental treated groups revealed markedly distended follicles and diffusely hyperplastic thyroid follicles lined with tall columnar epithelial cells. Their colloids are vacuolated with scalloped edges. An increase in serum FT3 and FT4 with decrease serum TSH was obtained in the treated group. Increased levels of G6PDH, LDH, ALP, MDA and decreased SOD were also observed. Activities of G6PDH, LDH, ALP, MDA, SOD, FT3, FT4 and TSH were highly significant when likened to the control group using one-way ANOVA statistical analytical method.
Conclusion: Results of this study showed the effects of cyanide cytotoxicity on the follicular cells of thyroid gland.
References
Lebot V. Tropical Root and Tuber Crops: Cassava, Sweet Potato, Yams and Aroids; CABI; Wallingford, UK, 2009.
Burns A, Gleadow R, Cliff J, Zacarias A, Cavagnaro T. Cassava: The drought, war and famine crop in a changing world. Sustainability 2010; 2:3572-3607.
Padmaja G. Cyanide detoxification in cassava for food and feed use. Critical Rev in Food Sci and Nutri 1995; 35:259–339.
Nartey F. Studies on cassava, Manihot utilissima Pohl—I. Cyanogenesis: The biosynthesis of linamarin and lotaustralin in etiolated seedlings. Phytochem 1968; 7:1307-1312.
Uyoh EA, Udensi O, natui V, Urua I. Effect of different processing methods on cyanide content of garri from four cultivars of cassava. J Food, Agric and Enviro 2007; 5(3&4):105-107.
Poulton JE. Localization and
Catabolism of Cyanogenic Glycosides.
In Cyanide Compounds in Biology; Evered D, Harnett S, Eds. Chichester, UK, John Wiley & Sons 1988; pp. 67-91.
Onabolu A. Cassava processing, Consumption and Dietary Cyanide Exposure. IHCAR Karohnska Institutet, Sweden, 2001; pp: 6-7.
Koch BM, Sibbeses O, Swain E, Kahn RA, Liangcheng D, Bak S, et al. Possible use of a biotechnological approach to optimize and regulate the content and distribution of cyanogenic glucosides in cassava to increase food safety. In: proc. of intern. Workshop on cassava safety. (Bokanga, M.; Esser, A.J.A.; Poulter, N; Rosling, H. and Tewe, O. eds) March 1-4 Ibadan Nigeria. Acta Hort 1994; 375: 45-60.
Achinewhu SC, Owuamanam CI.
Garification of five improved cassava cultivars, physicochemical and sensory properties of gari yield. Afr J Root Tuber Crops 2001; 4: 18-21.
Pearce LL, Bominaar EL, hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxillary substrate nitric oxide: an endogenous antidote to cyanide poisoning. J Biol Chem 2003; 278: 52135-52145.
Hariharakrishan J, satpute RM, Prasad GBKS, Bhattacharya R. Oxidative stress mediated cytotoxicity of cyanide in LLC-MK2 cells and its attenuation by alpha-ketoglutarate and N-acetyl cystein. Toxico Lett 2009; 185(2): 132141.
Oke OL. Processing and detoxifications of cassava. In: Delange F, Ahluwalia R, editors. Cassava toxicity and thyroid: research and public health issues. Ottawa, Canada: International
Development Research Centre (IDRC207e) 1982; pp. 129-33.
Cliff J, Lundquist P, Rosling H, Sobro B, Wide L. Thyroid function in a cassavaeating population affected by epidemic spastic paraparesis. Acta Endocrinol 1986; 113:523–528.
Hill RN, Erdreich LS, Paynter OE. Thyroid follicular cell carcinogenesis. Fundam. App.l Toxicol. 1989; 12(4): 629–697.
Delange F. Cassava cyanogenesis and iodine deficiency disorders. In: Bokanga, M., Essers, S.A.J.A., Poulter, N., Rosling, H and Tewe, O (eds.).
International workshop on cassava safety. Working Group on Cassava Safety (WOCAS). Acta Hort 1994; 289293.
FSANZ (Food Standards Australia New Zealand). Final assessment report proposal P257. Advice on the preparation of cassava and bamboo shoots. Report Number 2-04. Canberra, 2004.
Nhassico D, Muquingue H, Cliff J, Cumbana A, Bradbury JH. Rising African cassava production, diseases due to high cyanide intake and control measures. J Sci Food Agric 2008; 88: 2043-2049.
Speijers G. Cyanogenic glycosides. World Health Organization. WHO Food addictive Series, Geneva, 1993; 30: 299337.
FAO/WHO codex Alimentarius commission. Codex standard for edible cassava flour-African regional standard. CX-CPL. Rome 1991; 92-99.
FAO/WHO. Joint FAO/WHO Food Standards Programme, Codex Alimentarious Commission, XII supplement 4 FAO. Rome, 1999; pp: 1245.
Cardoso AP, Mirione E, Ernesto M, Massaza F, Cliff J, et al. Processing of cassava roots to remove cyanogens. J Food Compos Anal 2005; 18: 451-460.
Owuamanan CI, Iwouno JO,
Ihediohanma NC, Barber LI. Cyanide Reduction, functional and Sensory Quality of Gari as Affected by pH, Temperature and Fermentation Time. Pak J Nutri 2010; 9(10); 980-986.
Akindahunsi AA, Grisson FE, Adewusi SR, Afolabi OA, Torimiro SE, Oke OL. Parameters of thyroid function in the endemic goitre of Akungba and OkeAgbe villages of Akoko area of southwestern Nigeria. Afri J of Medicine and Medical sci. 1998; 27(3-4): 239242.
NAS (National Academy Sciences). “Guide for the Care and Use of Laboratory Animals” Institute for Laboratory Animal Research. Eight Edition. Washington, DC, National Academies Press, 2011.
Varone JC, Warren TN, Jutras K, Molis J, Dorsey J. Report of the investigation committee into the cyanide poisonings of Providence firefighters. New
Solution 2008; 18(1): 87-101.
Kiernan JA. Histological and Histochemical methods: Theory and Practice, 2nd ed. Oxford, Pergamon Press, 1990
Pearse AGE. The periodic Acid-Schiff Reaction, Broadsheet No. 26 (new series), The Association of Clinical Pathologist: 1959.
Kletzien RF, Harris PK, Foellmi LA. Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients and oxidant stress. FASEB J 1994; 8(2): 174-181.
Wei Bhaar D, Grossau E, Faderal B.
Normal ranges of alpha HBDH, LDH, AP and LAP as measured with substrateoptimated test charges. Med Welt 1975; 26: 387-392.
Babson LA, Greeley SJ, Coleman CM, Philips GD. Clin Chem 1966; 12: 482490.
Pasha KV, Sadasivadu B. Intracellular content thiol compounds, TBA reactive substances and gammaglutamyl tranpeptidase rat brain during anoxia. Neurosci Lett 1984; 46:209-214.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Euro J Biochem 1974; 47: 469-474.
Wild D. Immunoassay Handbook,
Stockton press, 1994; 339.
Lee RH, Spencer CA, Mestman JH. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol 2009; 200(3): 260.el-6.
Fisher DA. “Physiological variations in thyroid hormones. Physiological and pathophysiological considerations.” Clin Chem 1996; 42: 135-139.
Stevens A, Lowe J. Human Histology, 3rd edition, Elsavier Mosby, 2005; pp 279.
Kumar V, Abbas AK, Fausto N. Robbin and Cotran pathologic basis of diseases, 7th edition, Elsevier Mosby, 2004; 24: 1173.
Peters AL, Van Noorden CJF (2009). Glucose-6-phosphate Dehydrogenase Deficiency and Malaria: Cytochemical Detection of Heterozygous G6PD Deficiency in Women. J Histochem Cytochem 2009; 57(11): 1003–1011.
Nayak B, Burman K. Thyroitoxicosis and thyroid storm. Endocrinol Metab Clin N Am 2006; 35: 663-686.
Coquelle N, Fioravanti E, Weik M, Vellieux F, Madern D. Activity, Stability and Structural Studies of Lactate Dehydrogenases Adapted to Extreme Thermal Environments. J Mol Biol 2007; 374: 547–562.
Way JL. Cyanide toxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol 1984; 24:451-481.
Ballantyne B. Toxicology of cyanides. In: clinical and Experimental
Toxicology of cyanides. Ballantyne B, Marrs TC (Eds). Bristol, John Wright, 1987 pp 41-126.
Salkowski AA, Penney DG. Cyanide poisoning in animals and humans. A review. Vet Hum Toxicol 1994; 36:455466.
Murray RK, Granner DK, Mayes PA, Rodwell VW. Harper's Illustrated Biochemistry. 26th Ed. New York, McGraw-Hill: 2003.
Niki E, Noguchi N,Tsuchihashi H. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr 1995; 62: S1322-S1326.
Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membrane. Science 1972; 175 (4023): 720-731.
Das K, Chainy GBN. Modulation of liver mitochondrial antioxidant defense system by thyroid hormone. Biochem Biophys Acta 2001; 1573: 1–13.
Maggi-Capeyron MF, Cases J, Badia E, Cristol JP, Rouanet JM, Besancon P. A diet high in cholesterol and deficient in vitamin E induces lipid peroxidation but does not enhance antioxidant enzyme expression in rat liver. J Nutr Biochem 2002; 13: 296-301.
Araujo ASR, Ribeiro MFM, Enzveiler A, Schenkel P, Fernandez TRG, Partata WA, et al. Myocardial antioxidant enzyme activities and concentration and glutathione metabolism in experimental hyperthyroidism. Mol and Cell
Endocrinol 2006; 249: 133-139.
Lampka M, Junik R, Nowicka A, Kopczynska E, Tyrakowski A, Odrowaz G. Oxidative stress markers during a course of hyperthyroidism. J Pol Endocrinol 2006; 57: 218-222.
Asayama K, Kato K. (1990). Oxidative muscular injury and its relevance to hyperthyroidism. Free Radic Biol Med 1990; 8(3): 293–303.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

