Quandary of Enterococci - their beneficial and detrimental roles in food, environment and medicine
Keywords:
Antibiotic resistance, enterococci, food safety, infections, lactic acid bacteria, probioticAbstract
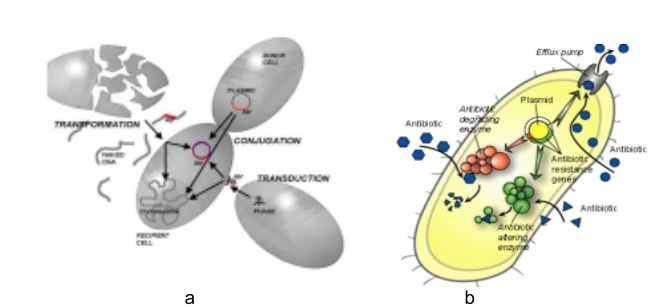
Enterococci are lactic acid bacteria that are widespread in nature; they were initially grouped with the genus Streptococcus. Enterococcus species are Grampositive cocci bacteria that have their applications in food, environment and health care. The presence of Enterococcus faecalis, in water for instance indicates faecal contamination and possible presence of pathogenic organisms. Oral application of probiotic enterococci confers health advantages while antihypertensive effect and amelioration of influenza infection by E. faecalis upon oral administration are beneficial to health. Improvement of intestinal ecosystem disturbed by antibiotic usage has been recorded. However, enterococci have ability to easily possess, amass and distribute genes that code for antibiotic resistance and pathogenic factors. They equally possess virulence factors among which are ability to hydrolyse gelatin, casein, haemoglobin and other bioactive peptides. They also cause agglutination and lyses of erythrocytes. The formation of biofilm on both biotic and abiotic surfaces is a universal survival strategy of enterococci. These organisms also induce the localization of cholesterol to phagosomes and also delay fusion upon infection. These negative attributes enable the organisms to acquire the potential to cause and inflict measurable harms in human. Furthermore, enterococci are intrinsically resistant to many antibiotics and possess ability to acquire and disseminate antibiotic resistance. They are of particular medical relevance because of their implication in both community- and hospital-acquired infections which could be transmitted through many sources. Therefore, man is still in a dilemma over the use of enterococci in food and other related industries owing to their ever increasing medical implications.
References
Busani L, Del Grosso M, Paladini C, Graziani C, Pantosti A, Biavasco F,
C a p r i o l i A . A n t i m i c r o b i a l susceptibility of vancomycinsusceptible and -resistant enterococci isolated in Italy from raw meat products, farm animals, and human infections. Int J Food Microbiol. 2004, 97:17-22.
Murray BE: The life and times of the Enterococcus. Clin Microbiol Rev 1990, 3(1):46-65.
David OM, Oluduro AO, Famurewa O. Property and antibacterial spectrum of partially purified enterocin produced by entrocinogenic Enterococcus faecalis isolated from the gut of cockroach. AU J. Technol. 2012, 16(2):74-80.
Schleifer KH, Kilpper-Balz R. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus
Enterococcus nom. rev. as
Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Bacteriol. 1984; 34:31-34.
Ghidan, A. Epidemiology of van A gene carrier enterococci: Molecular characterisation, antibiotic sensitivity and phylogenetic relationship of
Hungarian isolates. Thesis. Institute of Medical Microbiology, Semmelweis University. 2007.
Martins MT, Sato MIZ, Alves MN, Stoppe NC, Prado VM. Sanchez PS. Assessment of microbiological quality for swimming pools in South America. Water Res. 1995, 29(10):2417-2420.
Kirschner AKT, Zechmeister TC, Kavka GG, Beiwl C, Herzig A, Mach RL, Farnleitner AH. Integral strategy for evaluation of fecal indicator performance in bird-influenced saline inland waters. Appl. Environ.
Microbiol. 2004, 70(12):7396-7403.
Ahmed W, Neller R, Katouli M. Host species-specific metabolic fingerprint database for enterococci and Escherichia coli and its application to identify sources of fecal contamination in surface waters. Appl. Environ. Microbiol. 2005, 71(8):4461-4468.
Edberg SC, Le Clerc H. Robertson J. Natural protection of spring and well drinking water against surface
microbial contamination: II, indicators and monitoring parameters for
parasites. Crit. Rev. Microbiol. 1997, 23:179-206.
World Health Organization, Guidelines for drinking-water quality, 3rd, Recommendations, vol. 1, WHO, Geneva, 2004.
Bitton JMG, Farrah SR, Ruskin RH, Butner J, Chou YJ. Survival of pathogenic and indicator organisms in ground water. Ground Water. 1983; 21:405-410.
Bitton G. Wastewater Microbiology, 3rd. Edition, Wiley-Liss, Hoboken, NJ. 2005, pp 746.
Quihley EMM. Prebiotics and probiotics: Their role in the management of gastrointestinal disorders in adults Nutr Clin Pract. 2012, 27(2):195-200.
Horvath A, Szajewska H. Probiotics, prebiotics, and dietary fiber in the m a n a g e m e n t o f f u n c t i o n a l gastrointestinal disorders. World Rev. Nutr. Diet. 2013, 108:40-48.
Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J.
Biotechnol. 2000, 84:197-215.
Franz CM, Stiles ME, Schleifer KH, Holzapfel WH. Enterococci in Foods – a conundraum for food safety. Int. J. Food Microbiol. 2003, 88:105-122.
Simonová M, Sirotek K, Marounek M, Lanková A. Lipolytic activity of potential probiotics enterococci and additive staphylococci. Acta Vet. Brno. 2008, 77:575-580.
Kročko M, Čanigová M, Ducková V, Artimová A, Bezeková J, Poston J. Antibiotic resistance of Enterococcus species isolated from raw foods of animal origin in South West part of Slovakia. Czech. J. Food Sci. 2011, 29(6):654-659.
Hayakawa K, Marchaim D, Martin ET, Tiwari N, Yousuf A, Sunkara B, et al.
Comparison of the clinical characteristics and outcomes associated with vancomycin-resistant
E n t e ro c o c c u s f a e c a l i s a n d vancomycin-resistant E. faecium bacteremia. Antimicrob. Agents Chemother. 2012, 56(5):2452-2458.
Oumer BA, Gaya P, Fernandez-Garcia E, Marciaca R, Garde, S, Medina M, et al. Proteolysis and formation of volatile compounds in cheese manufactured with a bacteriocinproducing adjunct culture. J. Dairy Res. 2001, 68:117-129.
Verraes C, Van Boxstael S, Van Meervenne E, Van Coillie E, Butaye P, Catry B, et al. Herman L. Antimicrobial resistance in the food chain: A Review. Int. J. Environ. Res. Public Health. 2013, 10:2643-2669.
Hugas M, Garriga M, Aymerich MT. Functionalty of enterococci in meat products. Int. J. Food Microbiol. 2003; 88: 223-233.
Cleveland J, Montville TJ, Nes IF, Chikindas ML. Bacteriocins: safe, natural antimicrobials for food preservation. Inter J. Food Microbiol. 2001, 71:1-20.
Riley MA, Wertz JE. Bacteriocin diversity: ecological and evolutionary perspectives. Biochimie. 2002, 84:357364.
Ben Embarek PK, Jeppesen V, Huss HH. Antibacterial potential of Enterococcus faecium strains isolated from sous-vide cooked fish fillets. Food Microbiol. 1994, 11(6):525-536.
Izquierdo E, Marchioni E, AoudeWerner D, Hasselmann C, Ennahar S. Smearing of soft cheese with Enterococcus faecium WHE 81, a multi-bacteriocin producer, against Listeria monocytogenes. Food
Microbiol. 2009, 26:16-20.
Jennes W, Dicks LM, Verwoerd DJ. Enterocin 012 a bacteriocin produced by Enterococcus gallinarum isolated from the intestinal track of ostrich. Appl. Microbiol. 2000, 2:349-357.
Drider D, Fimland G, Hechard Y, McMullen LM, Prévost H. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 2006, 70:564-82.
Line JE, Svetoch EA, Eruslanov BV, Perelygin VV, Mitsevich VV,
Mitsevich IP, et al. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob. Agents
Chemother. 2008, 52:1094-1100.
Van Tyne D, Martin MJ, Gilmore MS. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins. 2013, 5:895-911.
Olawale AK, David OM, Oluyege AO, Famurewa O. Potential pathogenic Enterococcus faecalis strains from ready-to-eat food outlets Intern. J. Trop. Dis. Health. In Press.
David OM, Famurewa O. Toward effective management of nosocomial infections in Nigerian hospitals- A
Review. Academic Arena. 2010,
(5):1-7.
Yamamoto Y, Togawa Y, Shimosaka M, Okazaki M. Purification and characterization of a novel bacteriocin produced by Enterococcus faecalis strain RJ-11. Appl. Environ
Microbiol.2003, 69:5746-5753.
Morrow LE, Goginari V, Malesker MA. Probiotic, prebiotic, and symbiotic in critically ill patients. Curr Opin Crit Care. 2012, 18:91-96.
Aderiye BI, David OM. Effects of fermented maize gruel (Ogi) on the haemato-biochemical profile of Wistar albino rats challenged with Shigella dysenteriae JBA 010. J. Food Studies. 2013a. 2(1):33-41.
Aderiye BI, David OM. Evaluation of prophylactic and therapeutic properties of ogi in rabbits infected with Salmonella Typhi. Int. Food Res. J. 2013b, 20(1):1857-1861.
Szajewska H. Microbiota modulation: can probiotics prevent/treat disease in pediatrics? Nestle Nutr. Inst. Worksop Ser. 2013, 77:99-110.
Udenigwe CC, Ejike CE, Quansah JK, Eze MO. Towards the management of hypertension: Modulation of the reninangiotensin system by food protein hydrolysates and peptides.
Biokemistri. 2011, 23(3):108-117.
Phelan M, Kerins D. The potential role of milk-derived peptides in cardiovascular disease. Food Funct. 2011, 2:153-167.
Wang C, Tian J, Wang Q. ACE inhibitory and antihypertensive properties of apricot almond meal hydrolysate. Eur. Food Res. Technol. 2011, 232:549-556.
Shimada T, Kondoh M, Motonaga C, Kitamura Y, Cheng L, Shi H, et al. Enhancement of anti-allergic affects me-diated by the kampo medicine Shoseiryuto (Xiao-Qing-Long-Tang in Chinese) with lysed Enterococcus faecalis FK-23 in mice. Asian Pacific J. Allergy Immunol. 2010, 28:59-66.
Shimada T, Cheng L, Enomoto T, Yang X, Miyoshi A, Shirakawa T. Lysed Enterococcus faecalis FK-23 oral administration reveals inverse association between tuberculin responses and clinical manifestations in perennial allergic rhinitis: a preliminary study. J. Invest. Allergol. Clin. Immunol. 2004, 14:187-92.
Maeda N, Nakamura R, Hirose Y,
Murosaki S, Yamamoto Y, Kase T. et al. Oral administration of heat-killed Lactobacillus plantarum L-137
enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int.
Immunopharmacol. 2009, 9(9):11221125.
Boltz DA, Aldridge JR Jr, Webster RG, Govorkova EA. Drugs in development for influenza. Drugs. 2010,
(11):1349-1362.
Izumo T, Maekawa T, Ida M, Noguchi A, Kitagawa Y, Shibata H, et al. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10(9):11011106.
Fukada K, Fujikura D, Nakayama Y, Kondoh M, Shimada T, Miyazaki T. Enterococcus faecalis FK-23 affects alveolar-capillary permeability to attenuate leukocyte influx in lung after i n f l u e n z a v i r u s i n f e c t i o n .
SpringerPlus. 2013, 2:1-10.
Droste JH, Wieringa MH, Weyler JJ, Nelen VJ, Vermeire PA, Van Bever HP. Does the use of antibiotics in early childhood increase the risk of asthma and allergic disease? Clin Exp Allergy. 2000, 30:1547-53.
McKeever TM, Lewis SA, Smith C, Collins J, Heatlie H, Frischer M, Hubbard R. Early exposure to infections and antibiotics and the incidence of allergic disease: a birth cohort study with the West Midlands General Practice Research Database. J. Allergy Clin. Immunol. 2002, 109:4350.
Celedon JC, Fuhlbrigge A, RifasShiman S, Weiss ST, Finkelstein JA. Antibiotic use in the first year of life and asthma in early childhood. Clin Exp Allergy. 2004, 34:1011-6.
Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, et al. Antibiotic exposure in early infancy and risk for childhood atopy. J.
Allergy Clin. Immunol. 2005,
:1218-24.
Zhang B, An Z, Shimada T, Liu S, Maeyama K. Oral administration of
Enterococcus faecalis FK-23 suppresses Th17 cell development and attenuates allergic airway responses in mice. Int. J. Mol. Med. 2012, 30:248254.
Shimada T, Cheng L, Shi HB, Hayashi A, Motonaga C, Tang J, et al. Invest.
Allergol. Clin. Immunol. 2007, 17(2):70-76.
Cebra JJ. Influences of microbiota on i n t e s t i n a l i m m u n e s y s t e m
development. Am. J. Clin. Nutr. 1999, 69:1046-1051.
Isolauri E, Sutas Y, Kankaanpaa P, Arvilommi H, Salminen S. Probiotics: effects on immunity. Am. J. Clin. Nutr. 2001, 73:444-450.
Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J. Effects of inactivated Enterococcus faecalis and mannan oligosaccharide and their combination on growth, immunity, and disease protection in rainbow trout. North Amer. J. Aquacult. 2013, 75:416-428.
Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hughes A. Immunomodulation of human blood cells following the ingestion of lactic acid bacteria. J. Dairy Sci. 1995. 78:491-497.
Kanasugi H, Hasegawa T, Goto Y, Ohtsuka H, Makimura S. Yamamoto T. Single administration of enterococcal preparation (FK-23) augments nonspecific immune responses in healthy dogs. Int. J. Immunopharmacol. 1997, 19:655-659.
Benyacoub J, Czarnecki-Maulden GL, Cavadini C, Sauthier T, Anderson RE, Schiffrin EJ, et al. Supplementation of food with Enterococcus faecium (SF68) stimulates immune functions in young dogs. Nutritional Immunol. 2003, 133:1158-1162.
Kayser FH. Safety aspects of enterococci from the medical point of view. Int. J. Food Microbiol. 2003, 88:255-262.
Boonanantanasarn K, Gill AL, Yap YS, Jayaprakash V, Sullivan MA, Gill SR. Enterococcus faecalis enhances cell proliferation through hydrogen peroxide mediated epidermal growth factor receptor activation. Infect. Immun. 2012, 80(10):3545-3558.
Weisser M, Oostdijk EA, Willems RJL, Bonten MJM, Frei R, Elzi1 L, et al.
Dynamics of ampicillin-resistant Enterococcus faecium clones
colonizing hospitalized patients: data from a prospective observational study. BMC Inf. Dis. 2012, 12:68:1-9.
Ubeda C, Bucci V, Caballero S, Djukovic A, Toussaint NC, Equinda M, et al. Intestinal microbiota containing Barnesiella species cures vancomycinresistant Enterococcus faecium colonization. Infect. Immun. 2013, 81(3):965-973.
Moellering RC. Emergence of Enterococcus as a significant pathogen. Clin. Infect. Dis. 1992, 14(6):1173-1176.
Papanicolaou GA. Nosocomial infections with vancomycin-resistant Enterococcus faecium in liver transplant recipients: risk factors for acquisition and mortality. Clin. Infect. Dis. 1996, 23:760-766.
Hammerum AM, Fussing V, Aarestrup FM, Wegener HC. Characterization of v a n c o m y c i n - r e s i s t a n t a n d vancomycin-susceptible Enterococcus faecium isolates from humans, chickens and pigs by RiboPrinting and pulsed-field gel electrophoresis. J. Antimicrob. Chemother. 2000, 45:677680.
Graninger W, Ragette R. Nosocomial bacteraemia due to Enterococcus faecalis without endocarditis. Clin. Infect. Dis. 1992, 15:49-57.
Patterson JE. An analysis of 110 serious enterococcal infections: Epidemiology, antibiotic susceptibility and outcome. Medicine. 1995, 74:191-
Torres C, Klibi N, Ben A, Lagha K, Slama B, Boudabous, A. Faecal enterococci from camels in Tunisia: species, antibiotic resistance and virulent genes. Vet. Rec. 2013, 172:213. doi:10.1136/vr.100910.
Murray BE. Diversity among multidrug-resistant enterococci. Emerg. Infect. Dis. 1998, 4:37-47.
Rybaka AD, Hall ME, Arias CA, Murray BE, Michael J. Evaluation of standard- and high-dose daptomycin versus linezolid against vancomycinresistant Enterococcus isolates in an in v i t r o p h a r m a c o k i n e t i c / pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 2012, 56(6):3174-3180.
Tsai H, Liao C, Chen Y, Lu C, HuaHuang C, Lu C, et al. Trends in susceptibility of vancomycin-resistant Enterococcus faecium to tigecycline, daptomycin, and linezolid and molecular epidemiology of the isolates: Results from the tigecycline in vitro surveillance in Taiwan (TIST) study, 2006 to 2010. Antimicrob. Agents Chemother. 2012, 56(6):34023405.
Patidar RK, Gupta MK, Singh V.
Phenotypic detection of virulence traits and antibiotic susceptibility of endodontic Enterococcus faecalis isolates. Amer. J. Microbiol. Res. 2013, 1(1):4-9.
Franz CMAP, Holzapfel WH, Stiles ME. Enterococci at the crossroads of food safety? Int. J. Food Microbiol. 1999, 47(1-2):1-24.
Gulhan T, Aksakal A, Ekin UH, Savasan S, Boynukara B. Virulence factors of Enterococcus faecium and Enterococcus faecalis strains isolated from humans and pets. Turk. J. Vet. Anim. Sci. 2005, 30:477-482.
Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009, 155:1749-1757.
Clewell DB. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev, 1981, 45:409-436.
Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, et al. Endocarditis and biofilmassociated pili of Enterococcus faecalis. J. Clin. Invest. 2006, 116(10):2799-2807.
Zhou X, Wang X, Guo B, Wang X.
(2013). Isolation and identification of Enterococcus faecalis and detection of its virulence factor genes in lambs presenting with encephalitis in Xinjiang province, China. Afr. J. Microbiol. Res. 7(20):2238-2244.
David OM, Alegbeleye M, Ayeni LE, Famurewa O. Virulence-markers distribution and antibiotic resistance in Enterococcus species isolated from a tertiary health care facility in Ekiti State, Nigeria. AU J. Technol. (In Press).
Kurl DN, Haataja S, Finne J.
Hemagglutination activities of group
B, C, D, and G streptococci:
Demonstration of novel sugar specific cell-binding activities in Streptococcus suis. Infection and Immunity 1989, 57, 384-389.
Carvalho MGS, Teixeira LM.
Hemagglutination properties of
Enterococcus. Curr. Microbiol. 1995, 30:265-268.
Elsner HA, Sobottka I, Mack D, Claussen M, Laufs R, Wirth R. Virulence factors of Enterococcus faecalis and Enterococcus faecium blood culture isolates. European J. Clin. Microbiol. Inf. Dis. 2000, 19:3942.
Clewell DB. Bacterial sex pheromoneinduced plasmid transfer. Cell. 1993, 73 9-12.
Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin. Microbiol. Rev. 2000, 13:513522.
Eaton TJ, Gasson MJ. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol. 2001, 67:1628-1635.
Franz CMAP, Muscholl-Silberhorn AB, Yousif NMK, Vancanneyt M, Swings J, Holzapfel WH. Incidence of virulence factors and antibiotic resistance among enterococci isolated from food. Appl. Environ. Microbiol. 2001, 67:4385-4389.
Semedo T, Santos MA, Lopes MFS, Marques JJF, Crespo, MTB, Tenreiro R. Virulence factors in food, clinical and reference enterococci: A common trait in the genus? Syst. Appl. Microbiol. 2003, 26:13-22.
Dupont H, Vael C, Muller-Serieys C. Prospective evaluation of virulence factors of enterococci isolated from patients with peritonitis: Impact on outcome. Diagn. Microbiol. Infect. Dis. 2008, 60:247-53.
Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J. Endod. 2002, 28:689-693.
Flemming HC, Wingender J. The biofilm matrix. Nature Rev. Microbiol. 2010, 8:623-633.
Baureder M, Reimann R, Hederstedt L. Contribution of catalase to hydrogen peroxide resistance in Enterococcus faecalis. FEMS Microbiol. Lett. 2012. 331:160-164
Bourgogne A, Singh KV, Fox KA, Pflughoeft KJ, Murray BE, Garsin DA: EbpR is important for biofilm formation by activating expression of the endocarditis and biofilmassociated pilus operon (ebpABC) of
Enterococcus faecalis OG1RF. J Bacteriol 2007, 189(17):6490-6493.
Mohamed JA, Huang DB. Biofilm formation by enterococci. J Med Microbiol. 2007, 56:1581-1588.
Thurlow LR, Thomas VC, Narayanan S, Olson S, Fleming SD, Hancock LE. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect. Immun. 2010, 78:4936-4943.
Frank KL, Guiton PS, Barnes AMT, Manias DA, Chang-Smith ON, Kohler PL, et al. AhrC and Eep are biofilm infection-associated virulence factors in Enterococcus faecalis. Infect. Immun. 2013, 81(5):1696-1708.
Savage VJ, Chopra I, O'Neill A. Staphylococcus aureus biofilm promote horizontal transfer of antibiotic resistance. Antimicrob. Agents Chemother. 2013, 57(4):19681970.
Lasa I. Towards the identification of the common features of bacterial biofilm development. Int. Microbiol. 2006, 9:21-28.
Koch S, Hufnagel M, Theilacker C, Huebner J. Enterococcal infections: host response, therapeutic, and prophylactic possibilities. Vaccine. 2004, 22:822-830.
De Fatima Silva Lopes M, Ribeiro T, Abrantes M, Figueiredo Marques JJ,
Te n r e i r o R , C r e s p o M T B .
Antimicrobial resistance profiles of dairy and clinical isolates and type strains of enterococci. Int. J. Food Microbiol. 2005, 103:191-198.
Waters CM, Wells CL, Dunny GM. The aggregation domain of aggregation substance, not the RGD motifs, is critical for efficient internalization by HT-29 enterocytes. Infect Immun. 2003, 71:5682-5689.
David OM, Oluduro AO, Shitu A,
O l o w e O A , F a m u r e w a O .
Identification of changes during infection with gelatinase-producing and gelatinase defective strains of Enterococcus faecalis using liveanimal model. Vet. Res. 2011, 4(4):126-132.
Dunny GM, Brown BL, Clewell DB.
Induced cell aggregation and mating in
Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc. Natl. Acad. Sci. USA 1978, 75:3479-34838.
Clewell DB. Sex pheromone systems in enterococci, p. 47–65 In: G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C. 1999.
Dunny GM, Craig RA, Carron RL, Clewell DB. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979, 2:454-465.
Jett BD, Jensen HG, Nordquist RE, Gilmore MS. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect. Immun. 1992, 60:2445-2452.
Schaberg DR, Culver DH, Gaynes RP. Major trends in the microbial etiology of nosocomial infection. Am. J. Med. 1991, 91(Suppl 3B):72s-75s.
Flaherty JP, Weinstein RA. Nosocomial infection caused by antibiotic-resistant organisms in the intensive-care unit. Infect. Control Hosp. Epidemiol. 1996, 17:236-248.
Gray JW, Pedler SJ. Antibiotic-resistant enterococci. J. Hosp. Infect. 1992, 21:1-14.
Lu C, Chuang Y, Tsao S, Chen Y, Liu Y, Chen W, et al. Trends in susceptibility of vancomycin-resistant Enterococcus faecium to tigecycline, daptomycin, and linezolid and molecular epidemiology of the isolates: Results from the tigecycline in vitro surveillance in Taiwan (TIST) Study, 2006 to 2010. Antimicrob. Agents Chemother. 2012, 56(6):3402-3405.
Noble MA, Isaac-Renton JL, Bryce EA, Roscoe DL, Roberts FJ, Walker M, et al. The toilet as a transmission vector of vancomycin-resistant enterococci. J. Hosp. Infect. 1998, 40:237-241.
Nworie O, Mbaba M, Chukwudi A, Oko I, Chukwudum SO, Agah VM, et
al. Antibiogram of bacteria islolated from automated teller machines within Abakalili metropolis. Amer. J. Infect.
Dis. 2012, 8(4):168-174.
Clewell DB, Gawron-Burke C. Conjugative transposons and the dissemination of antibiotic resistance in streptococci. Annu Rev Microbiol. 1986; 40:635-659.
Bjorkeng EK. On mobile genetic elements in enterococci; Adding more facets to the complexity. Ph. D. Thesis.
Department of Medical Biology, University of Tromso, 2010.
Murray BE: Enterococci. Infectious diseases W. B. Saunders Company, Philadelphia, PaGorbach SL, Bartlett JG, Blacklow NR , 2 1998, 17231730.
Hummel A, Holzapfel WH, Franz CMAP. Characterisation and transfer of antibiotic resistance genes from enterococci isolated from food. Syst.
Applied Microbiol. 2007, 30:1-7.
S c h l i e v e r t P M , G a h r P J , Assimacopoulos AP, Dinges MM, Stoehr JA, Harmala JW, et al. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect. Immun. 1998, 66:218-223.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

