Evaluation of diagnostic utility and performance of rapid SARS-CoV- 2 antigen detection assay in comparison with Real-Time RT-PCR in Kolkata, India
Keywords:
ARDS, COVID-19, Rapid antigen, RT-PCR, SARS-CoV-2Abstract
Background: COVID-19 has so far affected millions of people in India. The present study was undertaken to find out the performance and reliability of rapid antigen test (RAT) in compared to reverse transcription polymerase chain reaction (RT-PCR).
Methods: The pre and existing medical conditions and clinical signs and symptoms were noted. The nasopharyngeal swab samples were taken for RAT, while both nasopharyngeal and oropharyngeal swab samples were mixed in a sterile viral transported medium (VTM) for RT-PCR. All patients were examined by RAT, while symptomatic negative in RAT were re-examined by RT-PCR.
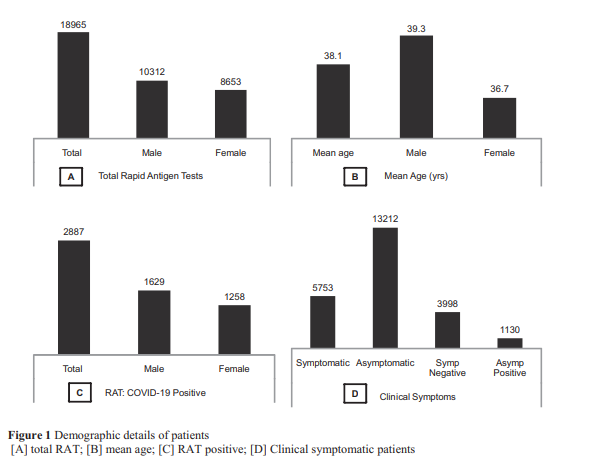
Results: Total 18,965 samples were examined by RAT and 3,998 samples by RT-PCR. Among them, only
5,753 patients (30.3%) were symptomatic and 1,757 patients (9.2%) were symptomatic positive. RAT showed overall 15.2% positive cases. Only 3.7% samples exhibited false negative results in RAT, which were found positive in RT-PCR. Interestingly, Ct (cycle threshold) values were >30 in all these samples.
Conclusion: Hence, specific antigen-based rapid diagnostic test (RDT) will be most useful and reliable among any other qualitative tests for screening purpose.
References
WHO Corona Virus (COVID-19) Dashboard. www.covid19.who.int
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early Transmission dynamics in Wuhan, China, of novel coronavirus infected pneumonia. New Engl J Med 2020; 382:1199-1207.
Ou X, Liu Y, Lei X, Li P, Mi D, Ren L. Characterization of spike glycoprotein of SARSCoV-2 on virus entry and its immune crossreactivity with SARS-CoV. Nat Commun 2020; 11:1620.
Zemlin E, Wiese OW. Coronavirus disease 2019 and the renin-angiotensin system: A closer look at the angiotensin-converting enzyme 2. Ann Clin Biochem 2020; 57:339-350.
WHO. Infection prevention and control during health care when COVID-19 is suspected 2020. www.who.int/publications/i/item/laboratorybiosafety-guidance-related-to-coronavirusdisease-2019-(covid-19)
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX. Clinical characteristics of coronavirus disease
in China. New Engl J Med 2020; 382:17081720.
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420-422.
Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473474.
Jose RJ, Manuel A. Covid-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med 2020; 8:e46-47.
Cevik M, Kuppalli K, Kindrachuk J, Peiris M. Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ 2020; 371:e3862.
Jiang C, Li X, Ge C, Ding Y, Zhang T, Cao S. Molecular detection of SARS-CoV-2 being challenged by virus variation and asymptomatic infection. J Pharm Anal 2021; 11:257-64.
Younes N, Al-Sadeq DW, Al-Jighefee H, Younes S, Al-Jamal O, Daas HI, et al. Challenges in laboratory diagnosis of the novel coronavirus SARS-CoV-2. Viruses 2020; 12:e582.
Das Mukhopadhyay C, Sharma P, Sinha K, Rajarshi K. Recent trends in analytical and digital techniques for the detection of the SARSCov-2. Biophys Chem 2021; 270:e106538.
Mak GC, Cheng PK, Lau SS, Wong KK, Lau CS, Lam ET, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol 2020; 129:e104500.
Kim D, Lee J, Bal J, Seo SK, Chong CK, Lee JH, et al. Development and clinical evaluation of an immunochromatography-based rapid antigen test
(GenBody™ COVAG025) for COVID-19 diagnosis. Viruses 2021; 13:e796.
Harrington A, Cox B, Snowdon J, Bakst J, Ley E, Grajales P, et al. Comparison of Abbott ID now and Abbott m2000 methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from symptomatic patients. J Clin Microbiol 2020; 58:e00798-20.
Dinnes J, Deeks JJ, Adriano A, Berhane S, Davenport C, Dittrich S, et al. Rapid, point-ofcare antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database of Syst Rev 2020; 8. Art. No.: CD013705.
CDC. Diagnostic tests for COVID-19 2020. w w w. c d c . g o v / c o r o n a v i r u s / 2 0 1 9 ncov/lab/testing.html.
Gupta A, Khurana S, Das R, Srigyan D, Singh A, Mittal A, et al. Rapid chromatographic immunoassay-based evaluation of COVID-19: A cross-sectional, diagnostic test accuracy study and its implications for COVID-19 management in India. Indian J Med Res 2021; 153:126-131.
Jacobs J, Kuhne V, Lunguya O, Affolabi D, Hardy L, Vandenberg O. Implementing COVID-19
(SARS-CoV-2) rapid diagnostic tests in subSaharan Africa: a review. Front Med 2020; 7:e557797.
Mak GC, Cheng PK, Lau SS, Wong KK, Lau CS, Lam ET, et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol 2020;129:104500
Cerutti F, Burdino E, Milia MG, Allice T, Gregori G, Bruzzone B, et al. Urgent need of rapid tests for SARS CoV-2 antigen detection: evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J Clin Virol 2020; 132:e104654.
Chaimayo C, Kaewnaphan B, Tanlieng N,
Athipanyasilp N, Sirijatuphat R, Chayakulkeeree M et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol J 2020; 17:e177.
Merino P, Guinea J, Munoz-Gallego I, GonzalezDonapetry P, Galan JC, Antona N, et al.
Multicenter evaluation of the Panbio™ COVID19 rapid antigen detection test for the diagnosis of SARS-CoV-2 infection. Clin Microbiol Infect 2021; 27:e758.
Osterman A, Baldauf H, Eletreby M, Wettenge JM, Afridi SQ, Fuchs T, et al. Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting. Med Microbiol Immunol 2021; 210:65-72.
Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-
9 p a t i e n t s . B M J 2 0 2 0 ;
https://doi.org/10.1101/2020.03.13.20035428
Mockel M, Corman VM, Stegemann MS,
Hofmann J, Stein A, Jones TC, et al. SARS-CoV2 antigen rapid immunoassay for diagnosis of
COVID-19 in the emergency department. Biomarkers 2021; 26:213-220.
Ristic M, Nikolic N, Cabarkapa V, Turkulov V, Petrovic V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS ONE 2001; 16:e0247606.
Nalumansi A, Lutalo T, Kayiwa J, Watera C, Balinandi S, Kiconco J, et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. IJID 2021; 104:282-286.
Peeling RW, Olliaro PL, Boeras DI, Fongwen N. Scaling up COVID-19 rapid antigen tests: promises and challenges. Lancet Infect Dis 2021; 29:e290.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

