Gastrointestinal motility and Intestinal structure following oral exposure to acrylamide in Wistar rats
Keywords:
Acrylamide, gastrointestinal tract, gastric emptying, intestinal motility, small intestinesAbstract
Introduction: Acrylamide, a byproduct of the cooking process, has been reported to be a toxicant with likely carcinogenic properties. Its impairment of gastric function has been previously reported. In this study its effects on gastrointestinal motility and intestinal structure was investigated in male Wistar rats.
Methods: Forty-five rats (120-180g) were divided into 3 equal groups (n=15) and treated p.o with either 0.2ml distilled-water, or acrylamide (7.5mg/kg and 15mg/kg respectively) for 28days. Thereafter, gastric emptying and intestinal motility was assessed. Intestinal structure (duodenum, jejunum and ileum), mucosal and intestinal cell counts were evaluated using histological techniques.
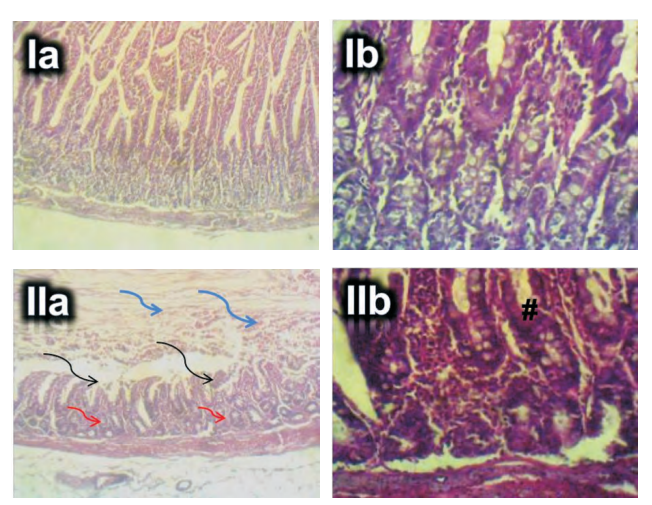
Results: Gastric emptying and intestinal transit time increased (p<0.05) in the experimental (acrylamidetreated; 7.5mg/kg and 15mg/kg) groups compared to control. Mucosal cell counts (duodenum, jejunum and ileum) and ileum intestinal cell counts (p<0.05) were reduced in the experimental groups compared to control. Compared to control, duodenal samples of the experimental groups showed severe coagulative necrosis and sloughing off of the villi, luminal filling with necrotic debris, disruption and necrosis of the crypts of Lieberkühn, moderate polymorphonuclear cell infiltration and vascular congestion. These pathologies albeit with less severity were also observed in the jejunum and ileum of acrylamide treated groups.
Conclusion: Increased oral exposure to acrylamide impairs gastric emptying, intestinal motility, mucus secretion and compromises digestive and absorptive functions of the small intestines, especially the duodenum. These observations may be ascribed to acrylamide-induced impaired neuronal signaling, autonomic neuropathy, oxidative stress, inflammation and cell necrosis.
References
Hall, JE. Gastrointestinal Physiology, In: Guyton and Hall Textbook of Medical Physiology (12th edition.) 2011. Philadelphia, PA: Elsevier, 753803.
Hammer AM, Morris NL, Earley ZM, Choudhry MA. The First Line of Defense: The Effects of Alcohol on Post-Burn Intestinal Barrier, Immune Cells, and Microbiome. Alcohol Res. 2015; 37(2):209-222.
Ige AO, Onwuka OM, Emediong IE, Odetola AO, Adele BO, Adewoye EO. Oral administration of acrylamide compromises gastric mucosal integrity in Wistar rats. J. Afr. Ass. Physiol. Sci. 2019; 7(1): 7-16.
Smith JL. The role of gastric acid in preventing foodborne disease and how bacteria overcome acid conditions. J Food Prot. 2003; 66(7):1292303. doi: 10.4315/0362-028x-66.7.1292.
Kitazawa T, Kaiya H. Regulation of Gastrointestinal Motility by Motilin and Ghrelin in Vertebrates. Front Endocrinol, 2019;10: 278. Doi: 10.3389/fendo.2019.00278
Takahashi T. Interdigestive migrating motor complex - its mechanism and clinical importance.
J Smooth Muscle Res. 2013;49:99-111. doi:10.1540/jsmr.49.99
Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review. J Agric Food Chem.
0 0 3 3 0 ; 5 1 ( 1 6 ) : 4 5 0 4 - 2 6 . d o i :
1021/jf030204+.
Exon JH. A review of the toxicology of acrylamide. J Toxicol Environ Health B Crit Rev.
0 0 6 ; 9 ( 5 ) : 3 9 7 - 4 1 2 . d o i :
1080/10937400600681430.
Mottram DS, Wedzicha BL, Dodson AT.
Acrylamide is formed in the Maillard reaction.
Nature. 2002; 419(6906):448-9. doi:
1038/419448a.
Palus K, Bulc M and Ca³ka J Effect of Acrylamide Supplementation on the Population of Vasoactive Intestinal Peptide (VIP)-Like Immunoreactive Neurons in the Porcine Small Intestine. Int. J.
M o l . S c i . 2 0 2 0 ; 2 1 : 9 6 9 1 ;
doi:10.3390/ijms21249691
Zödl B, Schmid D, Wassler G, Gundacker C, Leibetseder V, Thalhammer T, Ekmekcioglu C. Intestinal transport and metabolism of acrylamide. Toxicology 2007; 232: 99–108.
Zenick H, Hope E and Smith MK. Reproductive toxicity associated with acrylamide treatment in male and female rats. J. Toxicol. Environ. Health, 1986; 17: 457.
Eman MA, Amany YMR. Some Studies On
Acrylamide Intoxication In Male Albino Rats. Egypt. J. Comp. Path. & Clinic. Path. 2008; 21(4): 222 – 245
National Research Council (US) Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA, 1996.
Droppleman DA, Gregory RL, Alphin RS. A simplified method for assessing drug effects on gastric emptying in rats. J Pharmacol Methods.
; 4(3):227-30. doi: 10.1016/01605402(80)90014-5.
Yeung C-K, McCurrie JR and Wood D. A simple method to investigate the inhibitory effects of drugs on gastric emptying in the mouse in vivo. J Pharmacol Toxicol Methods 2001; 45: 235–240.
Sandhiya S, Dkhar SA, Krishna PRM,
Ramaswamy S. Role of ion channel modifiers in reversal of morphine-induced gastrointestinal inertia by prokinetic agents in mice. Indian J Exp Biol. 2008; 46: 60 – 65.
Tembhurne SV, Sakarkar DM. Effect of Murraya Koenigii leaves Extracts on Gastrointestinal motility: Involving Calcium Channel Innervation in Mice. Arch Pharm Sci & Res 2009; 1(2): 189 193.
Vigueras R.M, Rojas-Castaneda J, Hernandez R, Reyes G. and Alvarez C. Histological
characteristics of the intestinal mucosa of the rat during the first year of life. Laboratory Animals Ltd. Lab Anim. 1999; 33, 393-400.
Tareke E, Rydberg P, Karlsson S, Eriksson M, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 2002;50:4998–5006.
Zamani E, Shokrzadeh M, Fallah M, Shaki F. A review of acrylamide toxicity and its mechanism.
Pharm Biomed Res 2017; 3(1): 1-7 .
doi:10.18869/acadpub.pbr.3.1.1
Rodríguez-Varón A, Zuleta J. From the physiology of gastric emptying to the understanding of gastroparesis. Rev Colomb. Gastroenterol. 2010; 25(2), 219-225.
Guy RJ, Dawson JL, Garrett JR, Laws JW, Thomas PK, Sharma AK, Watkins PJ. Diabetic gastroparesis from autonomic neuropathy: surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg
Psychiatry. 1984; 47(7):686-91. doi:
1136/jnnp.47.7.686.
Oh JH, Pasricha PJ. Recent advances in the pathophysiology and treatment of gastroparesis. J Neurogastroenterol Motil. 2013; 19(1):18-24. doi:10.5056/jnm.2013.19.1.18
Bashashati M, McCallum RW. Is Interstitial Cells of Cajal?opathy Present in Gastroparesis?. J Neurogastroenterol Motil. 2015; 21(4):486-493. doi:10.5056/jnm15075
Bhetwal BP, An C, Baker SA, Lyon KL, Perrino BA. Impaired contractile responses and altered expression and phosphorylation of Ca 2+ sensitization proteins in gastric antrum smooth muscles from ob/ob mice. J Muscle Res Cell Motil. 2013; 34(2):137-49. doi: 10.1007/s10974013-9341-1.
Sullivan A, Temperley L, Ruban A. Pathophysiology, Aetiology and Treatment of Gastroparesis. Dig. Dis Sci. 2020; 65(6):1-17.
doi: 10.1007/s10620-020-06287-2
Sanders KM, Koh SD, Ro S, Ward SM.
Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol. 2012; 9(11):633-645. doi:10.1038/nrgastro.2012.168
Sickles DW, Stone D, Friedman MA.. Fast axonal transport: a site of acrylamide neurotoxicity? Neurotoxicology, 2002; 23:223–251.
LoPachin RM, Barber DS. Synaptic cysteine sulfhydryl groups as targets of electrophilic neurotoxicants. Toxicol Sci. 2006; 94:240–255.
LoPachin RM, Barber DS, Gavin T. Molecular mechanisms of the conjugated alpha, betaunsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci. 2008; 104(2):235-49. doi:
1093/toxsci/kfm301.
LoPachin RM, Gavin T. Molecular Mechanism of Acrylamide Neurotoxicity: Lessons Learned from Organic Chemistry Environ Health Perspect
0 1 2 ; 1 2 0 : 1 6 5 0 – 1 6 5 7 . http://dx.doi.org/10.1289/ehp.1205432.
Rao JN, Wang JY. Regulation of Gastrointestinal Mucosal Growth. San Rafael (CA): Morgan & Claypool Life Sciences; 2010. Intestinal Architecture and Development. Available from: https://www.ncbi.nlm.nih.gov/books/NBK5409
/
Umar S. Intestinal stem cells. Curr Gastroenterol Rep. 2010;12(5):340-348. doi:10.1007/s11894010-0130-3.
Ahmed M, Ahmed S. Functional, Diagnostic and Therapeutic Aspects of Gastrointestinal Hormones. Gastroenterology Res.
;12(5):233-244. doi:10.14740/gr1219.
El-Mehi AE and El-Sherif NM (2015). Influence of acrylamide on the gastric mucosa of adult albino rats and the possible protective role of rosemary. Tissue Cell. 47(3): 273-83. doi:
1016/j.tice.2015.03.005.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

