Exogenous Calcium Fibrin Sealant Loaded with Ageratum conyzoides Linn. Bactericidal Plant Extract: Equilibrium between a Biochemical Activator and Phytochemicals Inhibitors of Thrombin
Keywords:
Wound healing, fibrin sealant, Ageratum conyzoides Linn, biomaterial, inhibitorsAbstract
Objective: It is of primary importance to develop wound healing sealant that prevent bacteria contamination and growth. We propose to formulate poor platelets plasma material supplemented with a bactericidal plant extract, Ageratum conyzoides Linn. (Asteraceae). Aqueous extract of this plant is used as a bactericide.
Methodology: Platelet-poor plasma (PPP) containing less than 10,000 platelets per μL were used for all experimentations. Physiological serum (NaCl 0.9%) had served to prepare calcium chloride (CaCl ) solutions at 2 2M, 4M, 6M, 8M and 10M. Clauss fibrinogen assays were done to determine fibrinogen concentrations in each plasma pocket. Clotting times were measured following the addition of the appropriate calcium chloride concentration to the plasma. Plant extract at a concentration of 250 mg/mL in physiological serum was also added to the plasma and followed by the clotting times measured.
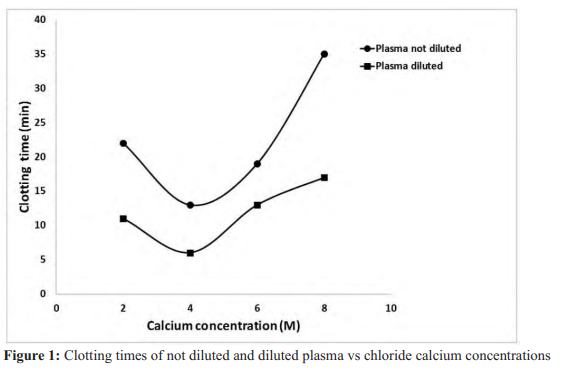
Results: Clauss fibrinogen assays reflected a global satisfactory fibrinogen concentration. Overall profile of the evolution of clotting time vs calcium concentrations adopts a curve form and the shortest clotting times are those obtained with 4M calcium concentration. However, the presence of extract deeply disturbed clotting process, as clotting times were extremely elongated.
Conclusion: Our objective was difficult to achieve because of the thrombin inhibitory side effects of the extract which were added to antithrombin III inhibitory effects in presence of excess calcium.
References
Burnouf, T., Goubran, H.A., Chen, T.-M., Ou, K.-L.,
Li, K., Diao, Y., Zhang, H., Wang, S., Zhang, Z., Yu,
El-Ekiaby, M., Radosevic, M., 2013. Blood-derived
B., Huang, S., Yang, H., 2011. Tannin extracts from
biomaterials and platelet growth factors in immature fruits of Terminalia chebula Fructus Retz.
regenerative medicine. Blood Reviews 27, 77–89. promote cutaneous wound healing in rats. BMC
Weisel, J.W., Litvinov, R.I., 2013. Mechanisms of Complementary and Alternative Medicine 11, 86. fibrin polymerization and clinical implications.
Akpalo A. Edefia, Douti F. Victoire, Layibo Yao,
Blood 121, 1712–1719.
Feteke Lochina, Karou Simplice, 2020. Plasma
Sproul, E., Nandi, S., Brown, A., 2018. Fibrin fibrin sealant exclusively based on exogenous biomaterials for tissue regeneration and repair, in: calcium and physiological thrombin: fitting and Peptides and Proteins as Biomaterials for Tissue understanding of an old approach. Annals of Regeneration and Repair. Elsevier, pp. 151–173. Biomedical Sciences 19, 1.
Janmey, P.A., Winer, J.P., Weisel, J.W., 2009. Fibrin 15. Miesbach, W., Schenk, J., Alesci, S., Lindhoff-Last, gels and their clinical and bioengineering E., 2010. Comparison of the fibrinogen Clauss assay applications. Journal of The Royal Society Interface and the fibrinogen PT derived method in patients
, 1–10. with dysfibrinogenemia. Thrombosis Research 126,
Radosevich, M., Goubran, H.I., Burnouf, T., 1997. e428–e433.
Fibrin sealant: scientific rationale, production 16. Shida, N., Kurasawa, R., Maki, Y., Toyama, Y., methods, properties, and current clinical use. Vox Dobashi, T., Yamamoto, T., 2016. Study of plasma
Sang 72, 133–143. coagulation induced by contact with calcium
Rybarczyk, B.J., 2003. Matrix-fibrinogen enhances chloride solution. Soft Matter 12, 9471–9476.
wound closure by increasing both cell proliferation 17. Stabenfeldt, S.E., Gourley, M., Krishnan, L., and migration. Blood 102, 4035–4043. Hoying, J.B., Barker, T.H., 2012. Engineering fibrin
A Okwori, C Dina, S Junaid, I Okeke, J Adetunji, A polymers through engagement of alternative Olabode, 2006. Antibacterial activities of Ageratum polymerization mechanisms. Biomaterials 33, conyzoides extracts on selected bacterial pathogens. 535–544.
The Internet Journal of Microbiology 4, 1. 18. Bijak, M., Ziewiecki, R., Saluk, J., Ponczek, M.,
Liu, L., Ma, H., Yang, N., Tang, Y., Guo, J., Tao, W., Pawlaczyk, I., Krotkiewski, H., Wachowicz, B., Duan, J., 2010. A Series of Natural Flavonoids as Nowak, P., 2014. Thrombin inhibitory activity of Thrombin Inhibitors: Structure-activity some polyphenolic compounds. Med Chem Res 23, relationships. Thrombosis Research 126, 2324–2337.
e365–e378. 19. Akpalo A. Edefia, Saloufou I. Kouassi, Eloh Kodjo
Khameneh, B., Iranshahy, M., Soheili, V., Fazly and Kpegba Kafui, 2020. Wound healing
Bazzaz, B.S., 2019. Review on plant antimicrobials: biomolecules present in four proposed soft aqueous a mechanistic viewpoint. Antimicrobial Resistance extractions of Ageratum conyzoides Linn.
& Infection Control 8, 118. International Journal of Biological and Chemical
Ndip R. N., A. N. Ajonglefac, T. Wirna, H. N. Luma, Sciences 14, 2.
C. Wirmum and S. M. N. Efange., 2009. In-vitro 20. Arif Budiman, Alfia Nur Azizah, Insan Sunan K, antimicrobial activity of Ageratum conyzoides 2018. Antibacterial activity of Ageratum conyzoides (Linn) on clinical isolates of Helicobacter pylori. L. Extract in gel dosage forms against African Journal of Pharmacy and Pharmacology Staphylococcus epidermidis and Propionibacterium
,11 pp. 585-592. Acne. Journal of Pharmacy Research 12, 584-588.
Singh S.B., Devi W.R., Devi W.I., Swapana N. and 21. Kaur Navpreet, Chaudhary Jasmine, Jain Akash and Singh C.B., 2013. Ethnobotany, phytochemistry Kishore Lalit, 2011. Stigmasterol: a comprehensive and pharmacology of Ageratum conyzoides Linn review. International Journal of Pharmaceutical
Sciences and Research IJPSR 2, 9: 2259-2265 25. Li, Q.-Q., Yang, Y.-X., Qv, J.-W., Hu, G., Hu, Y.-J.,
Oliva, M.L.V., Sampaio, M.U., 2009. Action of plant Xia, Z.-N., Yang, F.-Q., 2018. Investigation of
proteinase inhibitors on enzymes of Interactions between Thrombin and Ten Phenolic
physiopathological importance. An. Acad. Bras. Compounds by Affinity Capillary Electrophoresis
Cienc 81, 615–621. and Molecular Docking. Journal of Analytical
Bijak, M., Bobrowski, M., Borowiecka, M., Methods in Chemistry 2018, 1–8.
Podsędek, A., Golański, J., Nowak, P., 2011. 26. Mozzicafreddo, M., Cuccioloni, M., Eleuteri, A.M.,
Anticoagulant effect of polyphenols-rich extracts Fioretti, E., Angeletti, M., 2006. Flavonoids inhibit
from black chokeberry and grape seeds. Fitoterapia the amidolytic activity of human thrombin.
, 811–817. Biochimie 88, 1297–1306.
Pawlaczyk, I., Czerchawski, L., Pilecki, W., Lamer- 27. D. Simoes, S.P. Miguel, M.P. Ribeiro, P. Coutinho,
Zarawska, E., Gancarz, R., 2009. Polyphenolic- A.G. Mendonça, I.J. Correia, 2018. Recent
polysaccharide compounds from selected medicinal advances on antimicrobial wound dressing: A
plants of Asteraceae and Rosaceae families: review. European Journal of Pharmaeutis and
Chemical characterization and blood anticoagulant activity. Carbohydrate Polymers 77, 568–575. Biopharmaceuticss 127, 130-141
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

