Knowledge, perception and determinants of utilization of pharmacovigilance services among healthcare practitioners in Abuja.
Keywords:
Pharmacovigilance, knowledge, Healthcare practitioners (HCP), Adverse Drug Reaction (ADR) reporting, determinants of utilizationAbstract
Objective: To determine knowledge, perception, practice and determinants of utilization of pharmacovigilance services among healthcare practitioners (HCP).
Methods: This was a cross sectional study of HCP. A self-administered structured questionnaire was developed and pretested before administration. The participants were selected randomly using a sampling interval of four. Three hundred and forty questionnaires were administered. Data obtained were analyzed using SPSS version 20.
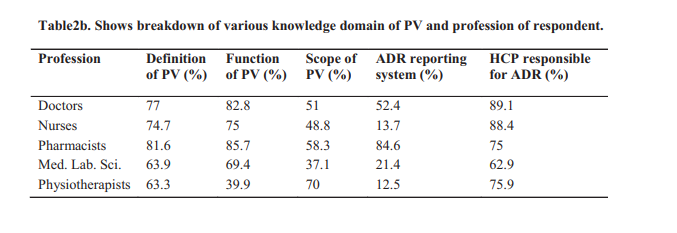
Results: The response rate was 89%, of which 42% were males. Majority of the respondents had good knowledge of pharmacovigilance (PV), however only about 11.8% have reported Adverse Drug Reactions (ADR), 26% have ever been trained on PV, 27% knew of the ADR reporting tool, the “yellow form”. The major reason for underreporting of PV was ignorance on the part of the practitioners.
Conclusion: The practice of PV among HCP is low, possible reasons include low training and poor knowledge of the ADR reporting tool. We recommend regular training of HCP to improve PV services.
References
World Health Organization. Essentials medicines and health products: pharmacovigilance [Internet]. Geneva: World Health Organization; 2004 Oct [cited 2018 Jul 9]. Available from: http://www.who.int/medicines/areas/quality_saf
ety/safety_efficacy/pharmvigi/en/
Naik P. The Future of Pharmacovigilance. J Pharmacovigil. 2015; 3: 159.
Oshikoya KA. Adverse drug reaction in children: types, incidence and risk factors. Nig J Paediatr. 2006;33:29-35.
Kongkaew C, Noyce PR, Ashcroft DM. Hospital admissions associated with adverse drug reactions: A systematic review of prospective observational studies. Ann Pharmacother. 2008;42(7):1017-25.
Patel KJ, Kedia MS, Bajpai D, Mehta SS, Kshirsagar NA, Googtay NJ. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the Medical emergency department of tertiary referral centre: a
prospective study. Clin Pharmacol. 2007;7(1):1-
Gupta SK. Text book of pharmacovigilance: Current Methods of Pharmacovigilance. New Delhi: Jaypee brothers Medical Publisher (P) Ltd; 2011. p. 28-38.
Subramaniyan G, Gunaseelan V, Kishtapati CR, Subrahmanyam DK, Chandrasekaran A. A survey on knowledge, attitude and practice of pharmacovigilance towards adverse drug reactions reporting among Doctors and Nurses in a Tertiary Care Hospital in South India. J Young Pharm. 2016;8(4):471-476
Fatemah A, Sherifah A, Eman A, Tania B,
Jacinthe L. Knowledge, attitude and practices of pharmacovigilance and adverse drug reaction reporting among pharmacists working in secondary and tertiary governmental hospitals in Kuwait. Saudi Pharm J. 2017;25(6):830-837.
Shaibu OB, Mohammad TU. Knowledge and attitude of physicians relating to reporting of adverse drug reactions in Sokoto, north western Nigeria. Ann. Afr. Med. 2011;10(1):13-18.
World Health Organization. The importance of pharmacovigilance: safety monitoring of medicinal products [Internet]. Geneva: World Health Organization; 2002 Jan [cited 2018 Jul 9].
A v a i l a b l e f r o m : http://apps.who.int/medicinedocs/en/d/Js4893e/.
Gupta SK, Nayak RP, Shivaranjani R, Vidyarthi SK. A Questionnaire study on the knowledge, attitude and the practice of pharmacovigilance among the healthcare professionals in a teaching hospital in South India. Perspect Clin Res. 2015;6(1):45-52.
Fadare J, Okezie O, Afolabi A. Knowledge, attitude and practice of adverse drug reaction reporting among healthcare workers in a tertiary center in Northern Nigeria. Trop J of Pharm Res. 2011;10(3):235-242.
Okechukwu R, Odinduka O, Ele G, Okonta J. Awareness, attitude, and practice of Pharmacovigilance among health care
professionals in Nigeria. Int. J hosp. res. 2013; 2(3):99-108.
Adedeji A, Fehintola A, Ibraheem A. Attitude and practice of doctors toward adverse drug reactions (ADRs) reporting in a Nigerian tertiary health facility. Ann. Ib Postgrad Med. 2013;11(3):7780.
Bello S, Umar M. Knowledge and attitude of physicians relating to reporting of adverse drug reactions in Sokoto, North-western Nigeria. Ann. Afr. Med. 2011 10(1): 13-8.
Singh A, Masuku M. Sampling techniques and determination of sample size in applied statistics research: an overview. Int. J. economics commerce manag. 2014;2(11):14-16.
Lokesh VR, Javeed SK, Mohanraj R, Padmanabha R. Assessment of knowledge, attitude and perception of pharmacovigilance and adverse drug reaction (ADR) reporting among the pharmacy students in south India. J Pharm. Biol. Sci. 2014;9(2):34-43.
Ebenebe UE, Ezeuko AY, Nnebu CC, Ugorji JO, Duru CB, Nwabueze AS. Adverse drug reaction reporting practices among health workers in Nnewi Nigeria. J int. res. Med.pharm. Sci. 2015;2(4):120-127.
Maniyan G. Gunaseelan V, Kishtapati C, Hmanyam D, Chandrasekaran A. A Survey on Knowledge, and Practice of Pharmacovigilance towards Adverse drug reactions reporting among Doctors and Nurses in a Tertiary Care Hospital in South India. J Young Pharm. 2016;8(4):471-476.
Su CJ, Su HY. Hospitals pharmacists' knowledge and opinions regarding adverse drug reaction r e p o r t i n g i n N o r t h e r n C h i n a .
Pharmacoepidemiol Drug Saf. 2010;19(3): 21722.
Hanafi S, Torkamandi H, Hayatshahi A, Gholami K, Javadi M. Knowledge, attitude and practice of nurse regarding adverse drug reaction reporting. Iran J Nurs Midwifery Res. 2012;17(1): 21-25.
Showande S, Fakeye T. The concept of adverse drug reaction reporting: awareness among pharmacy students in a Nigerian university. Internet journal of medical update. 2013;24(8)30.
Khan S, Goyal C, Chandel N, Rafi M.
Knowledge, attitudes, and practice of doctors to adverse drug reaction reporting in a teaching hospital in India: An observational study. J Nat Sc Biol Med.2013;4(1):191-6.
Bäckström M, Mjörndal T. A small economic inducement to stimulate increased reporting of adverse drug reactions – A way of dealing with an old problem? Eur J Clin Pharmacol.
;62:381-5.
Osakwe A, Ibrahim O, Adebowale JA, Abisola A, Iretiola F. Impact of training on Nigerian healthcare professional' knowledge and practice of pharmacovigilance. Int J Risk Saf Med 2013;25:219-227.
Mala K, Suresh B. Extent of under reporting of adverse drug reactions (ADRs) in India: Evaluation using logistics regression analysis (LRA) model. J Clin Trials. 2014; 4:1.
World Health Organization. The safety of medicines in public health programmes.
Pharmacovigilance: an essential tool [Internet]. World Health Organization; 2006 April [cited 2 0 1 8 J u l 9 ] . A v a i l a b l e f r o m :
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

