Butanol extract of Manihot esculenta leaf modulated cigarette butt leachate-mediated liver mitochondrial membrane permeabilization and its functional capacity
Keywords:
Manihot esculenta leaf extract, cigarette butt leachate, mitochondrial membrane permeability transition pore, liver functionAbstract
Objective: The experiment was designed to investigate the modulatory effects of the butanol extract of Manihot esculenta leaf, in vivo, and against the effects of cigarette butt leachate, in vitro on liver mitochondrial membrane permeability transition (MMPT), and its possible hepatoprotection in female Wistar rats.
Methods: The powdery form of fresh, air-dried and pulverized M. esculenta leaves were extracted in 95% butanol using Soxhlet apparatus and concentrated to a sticky mass using rotary evaporator. Ethical approval for animal use was obtained from Ladoke Akintola University of Technology, Osogbo, Nigeria (LTH/EC/2014/10/237). Twelve Wistar rats were randomly assigned into 2 groups of 6 rats each, and a separate group of 4 rats were used for the in vitro study. The first set of rats was orally treated daily with the extract for 21 days. Following an overnight fast, the rats were sacrificed by cervical dislocation and the liver mitochondria isolated by standard methods. Mitochondrial swelling and liver function assays were done spectrophotometrically.
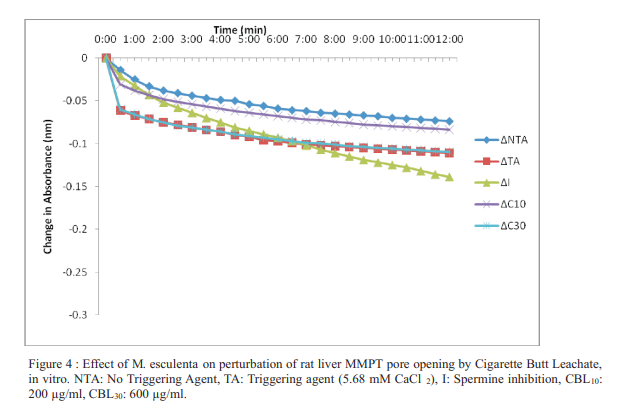
Results: In vivo, the extract significantly induced low amplitude pore opening in the absence of exogenous Ca2+; whereas when it was present, the extract slightly inhibited same. Two concentrations of cigarette butt leachate caused appreciable low amplitude swelling in control and treated animals in the presence of Ca2+; in the absence of which highly significant inhibitory effects were observed at the same concentrations, in vitro. Significant increases were also recorded for AST, ALT, bilirubin, total protein and ALP, in vivo.
Conclusion: The extract showed promise for hepato-protection against cigarette butt leachate toxicity in the liver of normal rats in the presence of exogenous Ca2+, in vitro.
References
Ujval A. and Jochen H. M. P. Anti-apoptotic BCL2 family proteins in acute neural injury. Front Cell Neurosci. 2014, 8: 281.
Concannon C. G., Tuffy L. P., Weisova P., Bonner H. P., Davila D., Bonner C. et al. AMP kinasemediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J. Cell Biol. 2010, 189, 83–94.
D'Orsi B., Bonner H., Tuffy L. P., Dussmann H., Woods I., Courtney M. J. et al. Calpains are downstream effectors of bax-dependent excitotoxic apoptosis. J. Neurosci. 2012, 32:1847–1858.
Kilbride S. M. and Prehn J. H. Central roles of apoptotic proteins in mitochondrial function.
O n c o g e n e 2 0 1 3 , 3 2 , 2 7 0 3 – 2 7 1 1
1038/onc.2012.348.
Dubois C., Fabien V. A., Natacha P. (2013). Targeting apoptosis by the remodelling of calcium-transporting proteins in cancerogenesis. FEBS Journal 280: 5500–5510.
Chucair A. J., Rotstein N. P., SanGiovanni J. P., During A., Chew E. Y., Politi L. E. Lutein a n d zeaxanthin protect photoreceptors from apoptosis induced by oxidative stress: relation with docosahexaenoic acid. Invest Ophthalmol Vis Sci. 2007, 48:5168–5177.
Lapidus R. G. and Sokolove P. M. Spermine inhibition of the permeability transition of isolated rat liver mitochondria: An investigation mechanism. Archives of Biochemistry and Biophysics 1993, 3061: 246-253.
Kroemer G. and Reed J. C. Mitochondrial control of cell death. Nat Med. 2000, 6:513–519.
Vieira H. L., Costantini P., Belzacq A. S., Larochette N., De Pablo M. A., Zamzami N. et al. Oxidation of a critical thiol residue of the adenine nucleotide translocator enforces Bcl-2independent permeability transition pore opening and apoptosis. Oncogene 2000, 19:307–314.
Zamzami N. and Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat Rev Mol Cell Biol 2001, 2:67–71
Wei Y., Lin X. and Zhu C. Chiral separation of some â-blockers using electrophoresis with dual cyclodextrin systems. Canadian Journal of Analytical Sciences and Spectroscopy 2005, 50. 3: 135–140.
Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem. J. 1999, 341: 233–249.
Woodfield K., Ruck, A., Brdiczka, D. and Halestrap, A. P. Direct demonstration of specific interactions between cyclophilin-D and the adenine nucleotide translocase confirm their role in the mitochondrial permeability transition. Biochemical Journal 1998, 336, 287–290.
Green D.R. and Kroemer G. The pathophysiology of mitochondrial cell death. Science 2004, 305: 626–629.
Anyasor G.N., Ajayi E.I.O., Saliu J.A., Ajagbonna O., Olorunsogo O. O. (2009). Artesunate opens mitochondrial membrane permeability transition pore. Ann Trop Med Public Health 2009, 2(2): 39-41.
Chipuk J., Moldoveanu T., Llambi F., Parsons M. and Green D. The BCL-2 family reunion. Mol Cell 2010, 37: 299–310.
Achidi A.U., Ajayi O.A , Bokanga M. and Maziya-Dixon B. The Use of Cassava Leaves as Food in Africa. Ecology of Food and Nutrition 2005, 44(6):423-435.
Res. J. of Health Sci. Vol 6(1), Jan./Mar., 2018 31
Parmar A., Sturm B. and Hensel O. Crops that feed the world: Production and improvement of cassava for food, feed, and industrial uses. Food Security 2017, 9(5):907–927
Fiedor J. and Burda K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6(2):466–488.
World Health Organization WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: World Health Organization 2008.
Vainio H. Is passive smoking increasing cancer risk? Scand. J Work Environ. Health 1987, 13(3):193–196.
Ozoh O. B., Akanbi M. O., Amadi C. E., Vollmer W. and Bruce N. The prevalence of and factors associated with tobacco smoking behavior among long-distance drivers in Lagos, Nigeria. Afr Health Sci. 2017, 17(3): 886–895.
Novotny T. E., Lum K. and Smith E. Cigarettes butts and the case for an environmental policy on hazardous cigarette waste. Int. J. Environ. Res. Public Health 2009, 6:1691–705.
Hoffmann D. and Hoffmann I. The changing cigarette, 1950–1995. J Toxicol Environ Health; 1997, 50:307–64.
Warne MStJ, Patra R. W. and Cole B. Toxicity and a Hazard Assessment of Cigarette Butts t o Aquatic Organisms [abstract]. Interact 2002Programme and Abstract Book. Sydney: The
Royal Australian Society Chemical Institute, the Australasian Society of Ecotoxicology and the International Chemometrics Society 2002, 192.
Micevska T., Warne MStJ and Pablo F. Variation in, and causes of, toxicity of cigarette butts to a cladoceran and microtox. Arch. Environ. Contam. Toxicol. 2006, 50: 205–12.
Moriwaki H., Kitajima S. and Katahira K. Waste on the roadside, 'poi-sute' waste: its distribution and elution potential of pollutants into environment. Waste Manag. 2009, 29:1192–1197.
Doll, R., Petro R. and Borelian J. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004, 328(7455):1519.
Oreskes N. and Conway E. M. Merchants of Doubt: How a Handful of Scientists Obscured the Truth on Issues from Tobacco Smoke to Global Warming. San Francisco, CA: Bloomsbury Press 2010. ISBN 1-59691-610-9.
Michaels D. Doubt is their product: how industry's assault on science threatens your health. Oxford [Oxfordshire]: Oxford University Press 2008, pp. 4–5. ISBN 0-19-530067-X.
Brandt A. M. The cigarette century: the rise, fall and deadly persistence of the product that defined America. New York: Basic Books, a member of the Perseus Books Group, 2007. ISBN 0-46507047-7.
Bjerregaard B. K., Raaschou-Nielsen O., Sørensen M., Frederiksen K., Tjønneland A., Rohrmann S. et al. The effect of occasional smoking on smoking-related cancers: in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Causes & Control 2006, 17 (10): 1305–1309.
Pell J. P., Haw S., Cobbe S., Newby D. E., Pell A. C., Fischbacher C et al.Smoke-free legislation and hospitalizations for acute coronary syndrome. The New England Journal of Medicine 2008, 359 (5): 482–491.
Winickoff J. P., Friebely J. and Tanski S. E. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics 2009, 123(1):e7479.
Sanz A., Caro P. and Barja G. Protein restriction without strong caloric restriction decreases mitochondrial oxygen radical production and oxidative DNA damage in rat liver. J Bioenerg. Biomembr. 2004, 36:542–552.
Lowry O. H., Rosebrough N. J., Farr A. L. and Randall R. J. Protein measurement with the Folin phenol reagent. J Biol Chem 1951,193:265-75.
Shahjahan M., Sabitha K. E., Jainu M. and Shyamala-Devi C. S. Effect of Solanum trilobatum against carbon tetrachloride-induced hepatic damage in albino rats. Indian J. Med. Res. 2004, 120: 194 – 198.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Journal of Health Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Research Journal of Health Sciences journal is a peer reviewed, Open Access journal. The Journal subscribed to terms and conditions of Open Access publication. Articles are distributed under the terms of Creative Commons License (CC BY-NC-ND 4.0). (http://creativecommons.org/licences/by-nc-nd/4.0). All articles are made freely accessible for everyone to read, download, copy and distribute as long as appropriate credit is given and the new creations are licensed under the identical terms.

